Protein Sample Preparation
advertisement
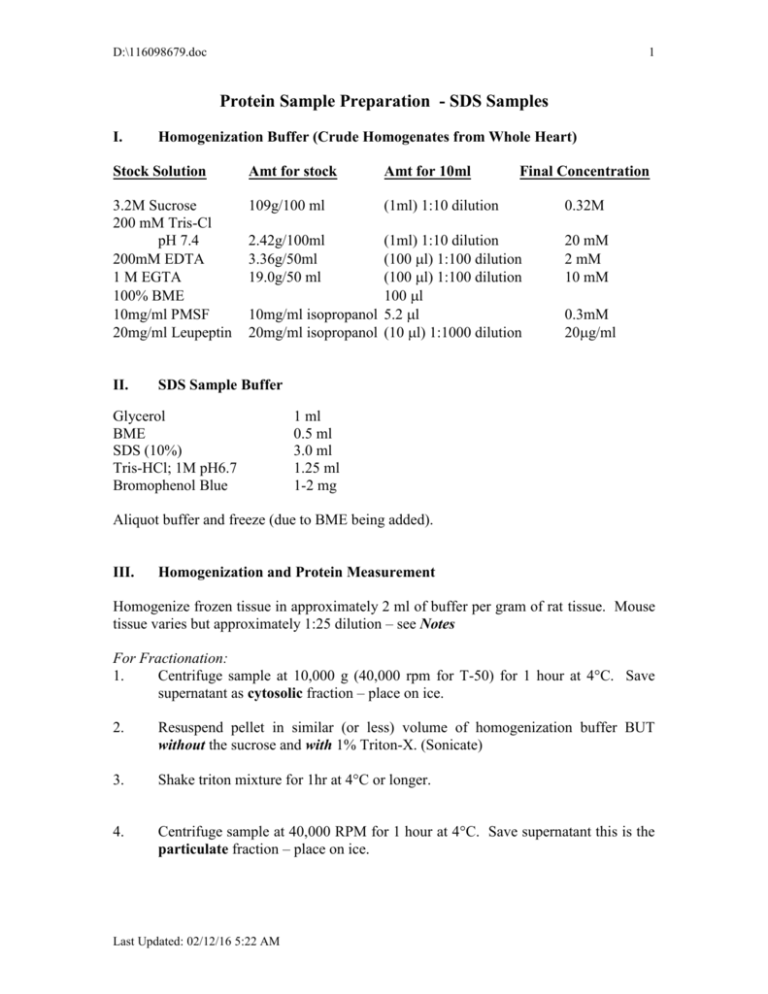
D:\116098679.doc 1 Protein Sample Preparation - SDS Samples I. Homogenization Buffer (Crude Homogenates from Whole Heart) Stock Solution Amt for stock Amt for 10ml 3.2M Sucrose 200 mM Tris-Cl pH 7.4 200mM EDTA 1 M EGTA 100% BME 10mg/ml PMSF 20mg/ml Leupeptin 109g/100 ml (1ml) 1:10 dilution II. 2.42g/100ml 3.36g/50ml 19.0g/50 ml Final Concentration (1ml) 1:10 dilution (100l) 1:100 dilution (100l) 1:100 dilution 100l 10mg/ml isopropanol 5.2l 20mg/ml isopropanol (10 l) 1:1000 dilution 0.32M 20 mM 2 mM 10 mM 0.3mM 20g/ml SDS Sample Buffer Glycerol BME SDS (10%) Tris-HCl; 1M pH6.7 Bromophenol Blue 1 ml 0.5 ml 3.0 ml 1.25 ml 1-2 mg Aliquot buffer and freeze (due to BME being added). III. Homogenization and Protein Measurement Homogenize frozen tissue in approximately 2 ml of buffer per gram of rat tissue. Mouse tissue varies but approximately 1:25 dilution – see Notes For Fractionation: 1. Centrifuge sample at 10,000 g (40,000 rpm for T-50) for 1 hour at 4°C. Save supernatant as cytosolic fraction – place on ice. 2. Resuspend pellet in similar (or less) volume of homogenization buffer BUT without the sucrose and with 1% Triton-X. (Sonicate) 3. Shake triton mixture for 1hr at 4°C or longer. 4. Centrifuge sample at 40,000 RPM for 1 hour at 4°C. Save supernatant this is the particulate fraction – place on ice. Last Updated: 02/12/16 5:22 AM D:\116098679.doc 2 Protein Measurement (Bradford Assay): Measure protein concentration in 30l (of 1:20) with BioRAD Assay Mixture (3 ml total volume). For mouse heart it may be necessary to increase sample volume to 20ul to bring into linear range of standard curve (Abs between 0.2 and 0.5). Linear relationship – Abs @595 vs. Protein concentration (g). y= 14.1x - 0.97 Calculate total protein concentration in samples and volume for 200 ug of protein for rat tissues and 800-1200 ug for mouse heart. *Tris interferes with this III. SDS Samples 1. Take desired g volume from stock homogenate and add 2.5-10% TCA to bring the total volume desired. 2. Spin at 3000 RPM for 15min 3. Decant and discard supernatant. Air dry pellet for 30-60 minutes (should be white precipitate). 4. Add 200 l of SDS stock, vortex and make sure pellet is dissolved (use approx 68ug/ul for mouse hearts). If sample is yellow add a drop of concentrated NaOH to titrate color to blue. 5. Boil samples for 5 minutes. 6. Cool samples to room temperature and freeze at –20C in Eppendorf tubes. Notes: 1. Heart samples obtained from mice should be diluted 1:25 with homogenization buffer. 2. Proteins should be precipitated with 2.5% TCA. Add SDS buffer for a final concentration of 6-8 g/ul. When SDS buffer is added it almost always requires titration with 1N NaOH – do this immediately and use only 1ul at a time. 3. Mouse heart samples for Western blot analysis should be run at 60-80 g of protein. Last Updated: 02/12/16 5:22 AM











