SDS PAGE electrophoresis
advertisement

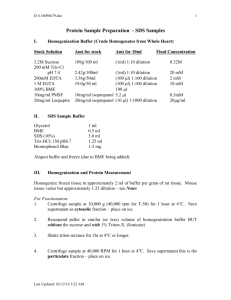
SDS PAGE electrophoresis Including: Sample preparation Electrophoresis assembly SDS-PAGE electrophoresis Materials: SDS PAGE Loading Buffer To make 10 mL of 4x stock: 2.5 ml 1M Tris-HCl pH 6.8 1g SDS 0.5 ml 0.3% Bromophenol Blue 4 ml of neat glycerol MilliQ water up to 10 ml Before use: add 50ul of neat β-mercaptoethanol into 1 ml of the stock solution 4X concentrations 250mM Tris-HCl pH 6.8 10% SDS 0.005% Bromophenol Blue 5% (approx. 0.7M) β-mercaptoethanol 40% Glycerol Final concentrations (1x) 62.5mM mM Tris-HCl pH 6.8 2.5% SDS 0.00125 % Bromophenol Blue 1.25% (approx. 0.18M)1% β-mercaptoethanol 10% Glycerol 5x Running buffer 5L : 75g Tris 360g Glycine 25g SDS MilliQ water up to 5L Protein markers: PageRuler Plus Pre-stained protein ladder x2 (Rainbow marker) or Protein Marker, Broad Range (P7702S) Protocols: Sample preparation: Mix your samples with loading buffer. Usually 5ug protein to up to 15ul MilliQ water and 5ul loading buffer. Boil them at 90 °C for 15 min or samples may also be treated at 37 °C for 2 h. Electrophoresis assembly Make sure a complete gelation of the stacking gel and take out the comb. Take the glass plates out of the casting frame and onto the electrode assembly and set them in the tank. Pour 1x Running Buffer into the inner chamber and in the outer chamber and remove the combs. SDS-PAGE electrophoresis Load prepared samples into wells and make sure not to overflow. Don't forget loading protein marker into the first lane. Attach the power leads and apply 100 volts to run the electrophoresis until the blue dye front reaches the bottom. . Note: Various factors affect the properties of the resulting gel. Higher concentration of ammonium persulfate and TEMED will lead to a faster gelation, on the other hand, a lower stability and elasticity. The optical temperature for gel gelation is 23°C-25°C. Low temperature will lead to turbid, porous and inelastic gels. The pH is better to be neutral and the gelation time should be limited in 20-30 min.