2. Protein sample preparation
advertisement

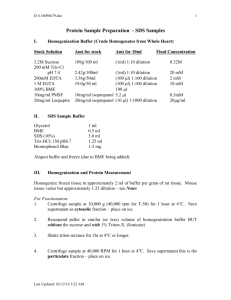
Protocol P2 May 15, 2009 PROTOCOL FOR PREPARATION OF PROTEIN SAMPLES 2.1 Materials needed 1. Extraction buffer 2. 5X SDS sample buffer 3. 1X SDS sample buffer 2.2 Instruments/Equipment needed 1. Centrifuge 2. Sonicator 3. Hot plate 2.3 Procedure 1. Withdraw 5ml of sample from cell culture. 2. Centrifuge the samples at 40 C for 10 minutes in a pre-chilled centrifuge. 3. Discard the supernatant and freeze the pellets in liquid nitrogen and store at -800 C until further processing. 4. Further processing – Thaw the pellet in ice, and 200ul of extraction buffer. Mix thoroughly. 5. Sonicate the samples for 5-6 times with an exposure of about 10 seconds each time. Keep the samples in ice to avoid damage to them by heat. 6. Centrifuge at 40 C for 5 minutes. 7. Separate the soluble fraction (supernatant) from the insoluble one (pellet). 8. To the soluble fraction - add 50ul of 5X sample buffer to about 250 ul of soluble fraction to make the total volume to 300 ul, thus diluting the 5X sample buffer to 1X. 9. To the insoluble fraction - add 200ul of 1X sample buffer. 10. Boil the samples for 5 minutes. 11. Centrifuge the samples at 13.8K rpm for 5 minutes. 12. Separate the supernatants from pellets (if any). 1 Protocol P2 May 15, 2009 13. If the supernatant is sticky and glutinous, repeat steps 5 and 6. Separate the supernatant from the pellets. 14. Store the soluble and insoluble fractions at 40 C. These are ready to be analyzed by SDS PAGE. 2.4 Extraction buffer composition – 1. 0.1 M HEPES-KOH pH 7.0 2. 20mM 2-mercapto ethanol 3. 0.1mg/ml Phenylmethyl-sulfonyl fluoride 4. 0.1% [v/v] Triton-X 5. 1mM EDTA 6. dH2O For calculations, refer to the calculation table and excel sheet. This buffer is to be made just before using it. 2.5 5X SDS sample buffer composition (10ml) 1. 0.5 M Tris-HCl pH 8.0 4.0ml 2. 20% SDS 2.5ml 3. DTT 386.0mg 4. Glycerol 2.5ml 5. BPB Pinch 6. dH2O 1.0ml 0 Store this buffer at 4 C. 2 Protocol P2 May 15, 2009 FLOWCHART FOR PROTEIN SAMPLE PREPARATION FOR SDS PAGE 5 ml culture sample Spin at 40C, 10’ Flash-freeze pellets, store at -800C 200 ul of extraction buffer Sonicate(10’’) – 5-6 times Spin at 40C, 5’ Separate soluble and insoluble fractions Soluble fraction – 50ul of 5X sample buffer Insol. fraction – 200ul of 1X sample buffer Boil the samples for 5’ Spin at 13.8K rpm, 5’ Collect supernatant from pellet (if any) Are the samples sticky? Sonicate(10’’) – 5-6 times Spin at 40C, 5’ Collect supernatant. Discard pellets Store sol./insol. Fractions at 40C. 3