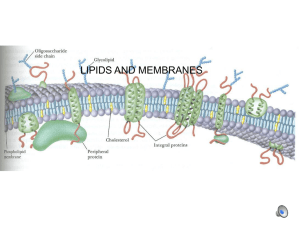
Identification and Characterization of Genes Conferring
advertisement

Identification and Characterization of Genes Conferring Reduced Saturate Oil in Sunflower (Helianthus annuus L.) James Gerdes1, Wenxiang Gao2, Robert Benson3, Angela Erickson4, and Charles Kahl5. 1 Dow AgroSciences, 2230 Hwy 75 North, Breckenridge, MN 56520, USA, jtgerdes@dow.com, 2 Dow AgroSciences, 9330 Zionsville Rd, Indianapolis, IN 46268, USA, wgao2@dow.com, 3 Dow AgroSciences, 2230 Hwy 75 North, Breckenridge, MN 56520, USA, rmbenson@dow.com, 4 Dow AgroSciences, 2230 Hwy 75 North, Breckenridge, MN 56520, USA, alerickson@dow.com, 5 Dow AgroSciences, 9330 Zionsville Rd, Indianapolis, IN 46268, USA, cjkahl@dow.com. ABSTRACT The reduction of saturated fatty acids in sunflower would greatly improve the quality resulting in healthier oil. The objectives of this research were to identify and characterize quantitative trait loci (QTL) that reduce the primary saturated fatty acids in sunflower, palmitic acid (C16:0) and stearic acid (C18:0). Accumulated gas chromatography (GC) data from diverse Dow AgroSciences (DAS) proprietary germplasm was methodically reviewed resulting in the identification of naturally occurring variants for both reduced C16:0 (lpm) and reduced C18:0 (lst). These variants were independently crossed to develop F2 mapping populations. The variants were also crossed together to evaluate combined effects in total saturate reduction. Major recessive QTL were identified for both lpm and lst using SSR markers. A QTL on LG5 explained 71.9% of the phenotypic variation for C16:0 and a QTL on LG17 explained 36.1% of the phenotypic variation for C18:0. Linked markers were identified and are being used to introgress these traits into elite inbred lines. Progeny from crosses between lpm and lst in a high oleic (Fad2-1) genetic background demonstrated total saturate levels as low as 2.33%. The total saturated fatty acid content of sunflower oil can be greatly reduced using the QTL identified in this study. The linked markers combined with GC enable rapid introgression of the traits. The non-GMO QTL identified in this work will allow development of parent lines and hybrids with significantly reduced total saturated fatty acid content. Fine mapping and candidate gene analyses for both lst and lpm are ongoing and will contribute to greater understanding of fatty acid biosynthesis in oilseeds. Key Words: Helianthus annuus, molecular marker, palmitic acid, quantitative trait loci, saturated fatty acid, stearic acid Introduction The discovery of cytoplasmic male sterility and genes for fertility restoration allowed the production of hybrid sunflower and promoted world sunflower production. The cultivated sunflower (Helianthus annuus L.) is a major worldwide source of vegetable oil. Seed oil from cultivated sunflower is comprised primarily of the saturated fatty acids palmitic (16:0) and stearic (18:0) acids, and the unsaturated fatty acids oleic (18:1), linoleic (18:2) and linolenic (18:3) acids (Dorrel and Vick 1997). Sunflower oil is premium oil because of its relatively high level of unsaturated fatty acid contents. Vegetable oils high in unsaturated fatty acids, such as oleic and linoleic acids, may have the ability to lower plasma cholesterol, reduce the risk of cardiovascular diseases (Mensink et al. 1994; Willett 1994). In contrast, saturated fatty acids, such as palmitic and stearic acids, have been reported in certain studies to contribute to an increase in the plasma cholesterol level, a factor in coronary heart disease (Willett 1994). Saturated fatty acids also have higher melting points in general than unsaturated fatty acids of the same carbon number, which contributes to cold tolerance problems in foodstuffs and can contribute to a waxy or greasy feel in the mouth during ingestion. Despite sunflower oil already having a high level of unsaturated fatty acids, it is still desirable to reduce its saturated fatty acid level for producing healthier oil. In addition, food products made from fats and oils having less than 3.5% saturated fatty acids will typically contain less than 0.5 gram saturated fat per serving and as a result can be labeled as containing “zero saturated fat” under current labeling regulations in the United States (www.fda.gov). Sunflower producing “zero saturated fat” oil is not currently available in the market. Palmitic and stearic acids are major saturated fatty acids in sunflower seeds and both are quantitatively inherited. Several quantitative trait loci (QTL) for palmitic and stearic acids have been reported (PérezVich et al. 2002; Ebrahimi et al. 2008). Genetic background and environment also play significant roles in palmitic and stearic acid inheritance (Pérez-Vich et al. 1999, 2002; Roche et al. 2006; Ebrahimi et al. 2008). Here, we present the results on the identification and characterization of genes conferring reduced palmitic and stearic acid content in sunflower and the application of those genes in the development of sunflowers producing unusually low saturated fatty acid oil. Materials and Methods Plant materials: Naturally occurring variants for low stearic acid (lst) and low palmitic acid (lpm) were identified based on accumulated gas chromatography (GC) data from diverse Dow AgroSciences (DAS) proprietary germplasm. These variants were independently crossed to develop F 2 mapping populations. Two F2 populations were generated, one from cross ONN687R x H757B/LS10670B-B-173-23-5 (simplified as ONN687R/H757B in this report) and the other from cross H757B/LS10670B-17-323-5-4 x H280R[1]/687R-1-8-1 (simplified as H757B/H280R in this report). H757B and H280R were the low stearic acid and low palmitic acid donors, respectively. Population ONN687R/H757B had 186 F2 individuals and was used to map low stearic acid QTLs, and population H757B/H280R had 188 individuals and was used to map low palmitic acid QTLs. Fatty acid analyses: Fatty acid profiles of oils extracted from individual F 2 half-seeds were quantified by gas chromatography (GC) and by using a proprietarily developed half-seed Fatty Acid Methyl Ester (FAME) analysis method. Seven fatty acids were measured, including palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), arachidic acid (20:0), behenic acid (22:0) and lignoceric acid (24:0). In brief, half-seeds were individually crushed with steal beads in 1 mL of heptane. After centrifugation at 4000 rpm for 10 min, a 200 µL aliquot of the supernatant was transferred into a 2.5 mL auto-sampler vial, diluted with 300 µL heptane, and followed by the addition of 40 µL of 2M KOH in methanol. The vials were capped and vortexed for 30 seconds. Samples were analyzed for fatty acid contents on an Agilent 6890 GC-FID (Agilent Technologies) equipped with a SGE BPx70 30 meter x 0.25 mm ID column and 0.25 µm film thickness (SGE Analytical Science). The initial oven temperature was 140 oC and maintained for one minute, followed by a rate increase of 20 oC /minute up to 250 oC. The oven was held at this temp for 0.5 minutes. The inlet was set to split ratio of 1:2 and a temperature of 280 oC. A constant column flow rate of 2.0 mL/min helium was maintained throughout the run. The detector was set to 300 oC with a constant carrier gas make up of 45.0 mL/min, fuel hydrogen flow of 30 mL/min, and oxidizer flow of 400 mL/min. An injection volume of 2 µL was used for all samples. DNA extraction and SSR marker analyses: The rest of the F2 half-seeds left from fatty acid analysis were grown in greenhouse. DNA was extracted from sampled leaf tissues. SSR markers were used to genotype the mapping populations. PCR reactions were performed in GeneAmp PCR System 9700 (Applied Biosystems) with dual-384 well block. PCR reaction volume was 8 µL containing 10 ng of DNA and 0.2 Units of Hot Start Taq DNA polymerase. The initial denaturing step was performed at 95 ºC for 12 minutes, followed by 40 cycles of 94 ºC for 5 seconds, 55 ºC for 15 seconds, 72 ºC for 30 seconds. The final extension was performed at 72 ºC for 30 minutes followed by 4 ºC forever. Forward primers of SSR markers were end-labeled with fluorescent dyes FAM, HEX, or NED. Multiplexed PCR products were resolved in ABI 3730XL DNA Analyzer. QTL Mapping: Join Map 3.0 (Van Ooijen and Voorrips 2001) was used to create a genetic linkage maps. Kosambi mapping function was employed (Kosambi 1944). MapQTL 4.0 (Van Ooijen et al. 2002) was used to map QTLs. Interval mapping method (Jansen 1993) was employed. Results and Discussions Mapping of QTLs for low stearic and low palmitic acids: Parental lines ONN687R, H757B, and H280R were screened with more than 400 SSR markers for identifying highly informative polymorphic ones to genotype F2 mapping populations. All 186 individuals from the ONN687R/H757B F2 mapping population were successfully genotyped with 76 selected informative polymorphic SSR markers and evaluated for the seven fatty acids listed in Table 1. The total saturated fats for this population averaged 5.79% with the lowest plant containing 3.77% total saturated fats which was approaching the threshold for United States Food and Drug Administration classification of a “zero saturated fat” oil (No Sat oil). LOD scores associated with a P < 0.05 and P < 0.01 as estimated from a 1,000 iteration permutation test (Churchill and Doerge 1993) for both the additive and additive + dominant statistical models were listed in Table 2. As expected, the LOD scores for the additive model were lower than that for the additive + dominant model as fewer parameters were estimated. One major QTL for low stearic acid was detected in the HA1875-HA1865 interval on linkage group 17, spanning 21.8 cM (Fig. 1). It explained 36.1% of the total phenotypic variation of stearic acid content in the population. Similarly, all 188 individuals from the H757B/H280R F2 mapping population were successfully genotyped with 49 informative polymorphic SSR markers and measured for all seven fatty acids, which resulted in the identification of one major QTL for low palmitic acid (Fig. 1; detailed statistic analysis data not shown). The low palmitic acid QTL was located in the 2 cM HA0908-HA0907 interval on linkage group 5 and explained 71.9% of the total phenotypic variation in palmitic acid content in the mapping population with a LOD score of 3.0. Table 1: Statistics on the ONN687R/H757B F2 mapping population (N= 186). Fatty Acid Palmitic Acid (C16:0) Stearic Acid (C18:0) Oleic Acid (C18:1) Linoleic Acid (C18:2) Arachidic Acid (C20:0) Behenic Acid (C22:0) Lignoceric Acid (C24:0) Total Saturates (C16-C18) Total Saturates (C16-C24) Mean (%) 3.12 1.89 91.02 2.95 0.11 0.51 0.16 5.01 5.79 Standard Deviation 0.64 0.63 4.74 4.43 0.15 0.43 0.15 0.87 0.88 Minimum (%) 2.20 0.49 48.43 0.77 0.00 0.00 0.00 3.17 3.77 Maximum (%) 4.94 3.47 95.39 44.26 0.64 1.31 0.56 7.87 8.08 Table 2: Experiment-wise permutation significance LOD score threshold. Fatty acid Fitting Trait as Additive Model Fitting Trait as Additive and Dominant Model Palmitic Acid Stearic Acid Oleic Acid Linoleic Acid Arachidic Acid Behenic Acid Lignoceric Acid Total Saturates (C16-C18) Total Saturates (C16-C24) LOD Score (P<0.05/P<0.01) 2.5/3.8 2.5/3.2 1.7/2.0 1.6/1.9 2.4/3.1 2.5/3.1 2.5/3.3 2.5/3.4 LOD Score (P<0.05/P<0.01) 3.2/4.1 3.2/3.8 2.6/3.1 2.6/3.0 3.2/3.9 3.5/12.0 3.2/4.4 3.2/3.9 2.5/3.3 3.3/4.0 Figure 1: Showing the low stearic acid QTL in the HA1875-HA1865 interval on linkage group 17 and the low palmitic acid QTL in the HA0908-HA0907 interval on linkage group 5. QTL intervals were filled with grey color. Development of reduced saturates sunflower: Seed fatty acid contents are complex traits in sunflower. Many genes/QTLs and environment factors are involved in the inheritance. For example, the dominant Ol mutation resulted in a significant increase of oleic acid content in sunflower seeds but seed oleic content was significantly affected by genetic backgrounds (Soldatov 1976; Urie 1985; Miller et al. 1987; Schuppert et al. 2006). To develop sunflower germplasm with reduced saturates, a series of hybridization were conducted to introgress and integrate the low stearic and low palmitic aicd QTLs identified above into various genetic backgrounds. Normal breeding techniques such as backcross breeding, mass and recurrent selections, and pedigree breeding, etc, were employed during breeding practice. To speed up breeding process and increase selection gains, marker assisted selection was employed. Flanking markers HA1875 and HA1865 were used for selection of the low stearic acid QTL, and HA0907 and HA0908 for selection of the low palmitic acid QTL. Additional markers relatively evenly distributed in the sunflower genome were also used to select the genetic background. In addition, fatty acid profile of oils measured by GC was also used during the selection process. Successfully, a series of sunflower lines producing unusually low levels of saturates have been developed at Dow AgroSciences (Table 3). As can be seen in Table 3, progeny from crosses between lpm and lst demonstrated total saturate levels as low as 2.33% in a high oleic (> 80%, Fad2-1) genetic background and < 3.5% in a NuSun (55-50% oleic) background. Stearic acid levels reached as low as 0.25%, and palmitic acid levels as low as 1.47%. Table 4 listed sunflower lines that had the lowest level of saturates observed at Dow AgroSciences, 0.23% for low stearic acid, 1.37% for low palmitic acid and 2.33% for total saturates. Interestingly, all three lowest levels occurred in the high oleic genetic background, which suggested important roles of the high oleic mutation for the achievement of extremely low levels of saturated fatty acids in sunflower seeds. The results demonstrated that the total saturated fatty acid content of sunflower oil could be greatly reduced by introgression of the two QTLs identified in this study into various genetic backgrounds such as high linoleic acid, medium oleic acid or high oleic acid backgrounds, and that the linked SSR markers together with GC technology enabled rapid and efficient introgression of the lst and lpm traits. The results also demonstrated the existence of interactions between the two QTLs and their genetic backgrounds on seed saturated fatty acid level. To further understand the genetic mechanisms of the lst and lpm QTLs, fine mapping and gene cloning of the two QTLs are currently ongoing. More tightly linked molecular markers to the QTLs or gene specific markers developed from the underlining genes could improve the efficiency of marker assisted selection during breeding practice, and the cloned genes could benefit other oil crops for producing healthier oils. Table 3: DAS sunflower germplasm with unusually low saturate levels (%). Germplasm NuSun/No Saturate NS1982.16/OND163R-1-05 NS1982.8 No Saturate/High Oleic NS1982.8-03 NS1982.8H117R[4]//H757B/LS10 670B///NS 1982.6-2-023-1-12-076 Low Saturate/Linoleic CND117R/NS1982.8-3-06 OI1601B[2]//H757B/LS10670B[1] //NS1982.6=B-3-04 CN2343B/4/CN2343B[2]//H757B/ LS10670B///NS1982.11#1#1-3-11 Low Stearic NS1982.8/OND163R-2-12-009 H117R[4]//H757B/LS10670B///NS 1982.6-2-023-1-12-038 OID263R/NS1982.8-4-12-002 Low Palmitic H251B[2]/IAST4=1=100//NS1982.16-11-39-041 NS1982.14-08 NS1982.16 Very High Oleic H117R[4]//H757B/LS10670B/NS1 982.6-2-023-1-12-076 NS1982.8/OND163R-2-12-059 ON3351B/NS1982.8-1-04 C16:0 C18:0 C18:1 C18:2 Total Saturates 2.29 2.09 0.65 0.55 67.37 79.40 28.19 15.99 3.48 3.10 1.60 1.63 0.37 0.41 95.13 94.81 1.48 1.26 2.33 2.48 1.79 0.29 95.30 0.84 2.57 5.29 3.76 0.73 0.80 18.19 34.97 74.43 58.62 6.41 5.29 3.13 2.07 36.03 56.65 6.23 2.75 1.90 0.25 0.27 92.95 95.03 1.99 1.00 3.43 2.65 3.08 0.27 93.54 1.48 3.87 1.47 2.59 92.59 0.65 5.42 1.51 1.52 2.24 1.05 92.84 94.37 1.35 0.85 4.90 3.39 1.79 0.29 95.30 0.84 2.57 1.87 2.04 0.44 0.50 95.22 95.20 0.97 0.70 2.76 3.08 Table 4: DAS sunflower germplasm with lowest level (%) of stearic acid, palmitic acid and total saturates. Germplasm NS1982.8/OND163R-12-90 H117R[4]//H757B/LS10670B-B-17-323=B1=2=16///NS1982.6-2-23.1-1 NS1982.8-03 C16:0 1.37 1.94 C18:0 1.70 0.23 C18:1 91.93 94.58 C18:2 2.83 1.80 Total Saturates 4.32 2.60 1.60 0.37 95.13 1.48 2.33 References Churchill, G.A., and Doerge, R.W. 1994. Empirical threshold values for quantitative trait mapping. Genetics 138: 963-971. Dorrel, D.G., and Vick, B.A. 1997. Properties and processing of oilseed sunflower. p. 709- 746. In: A.A. Schneiter (ed.), Sunflower technology and production. Crop Sci. Soc. Amer, Madison, WI, USA. Ebrahimi, A., Maury, P., Berger, M., Grieu, P., and Sarrafi, A. 2008. QTL mapping of seed-quality traits in sunflower recombinant inbred lines under different water regimes. Genome 51: 599-615. Jansen, R.C. 1993. Interval mapping of multiple quantitative trait loci. Genetics 135: 205-211. Kosambi, D.D. 1944. The estimation of map distances from recombination values. Ann Eug. 12:172-175. Mensink, R.P., Temple, E.H.M., Hornstra, G. 1994. Dietary saturated and trans fatty acids and lipoprotein metabolism. Ann. Med. 26: 461-464. Miller, J.F., Zimmerman, D.C., and Vick, B.A. 1987. Genetic control of high oleic acid content in sunflower oil. Crop Sci. 27: 923-926. Pérez-Vich, B., Fernández-Martínez, J. M., Grondona, M., Knapp, S. J., and Berry, S. T. 2002. StearoylACP and oleoyl-PC desaturase genes cosegregate with quantitative trait loci underlying high stearic and high oleic acid mutant phenotypes in sunflower. Theor. Appl. Genet. 104: 338-349. Pérez-Vich, B., Garcés, R., and Fernández-Martínez, J.M. 1999. Epistatic interaction among loci controlling the palmitic and the stearic acid levels in the seed oil of sunflower. Theor. Appl. Genet. 100: 105-111. Roche, J., Bouniols, A., Mouloungui, Z., Barranco, T., and Cerny, M. 2006. Management of environmental crop conditions to produce useful sunflower oil components. Eur. J. Lipid Sci. Technol. 108: 287-297. Schuppert, G.F., Tang, S., Slabaugh, M.B., and Knapp, S.J. 2006. The sunflower high-oleic mutant Ol carries variable tandem repeats of FAD2-1, a seed-specific oleoyl-phosphatidyl choline desaturase, Molecular Breeding 17: 241–256. Soldatov, K.I. 1976. Chemical mutagenesis in sunflower breeding. p. 352-357. In Proc. 7th Intl. Sunflower Conf., Krasnodar, USSR (Vlaardingen, the Netherlands: Intl. Sunflower Assoc.). Urie, L.A. 1985. Inheritance of high oleic acid in sunflower. Crop Sci. 25: 986-989. Van Ooijen, J.W., and Voorrips, R.E. 2001. JoinMap Version 3.0, Software for the calculation of genetic linkage maps. Plant Research International, Wageningen, the Netherlands. Van Ooijen, J.W., Boer, M.P., Jansen, R.C., Maliepaard, C. 2002. MapQTL 4.0, Software for the calculation of QTL positions on genetic maps. Plant Research International, Wageningen, the Netherlands. Willett, W.C. 1994. Diet and health: what should we eat? Science 264: 532-537.