Molecular Luminescence Spectrometry
Chapter 15- Molecular Luminescence Spectrometry
The following optical methods are described: molecular fluorescence, phosphorescence, and chemiluminescence. In each, molecules of the analyte are excited to give a species whose emission spectrum provides information for qualitative or quantitative analysis.
The methods are known as molecular luminescence procedures.
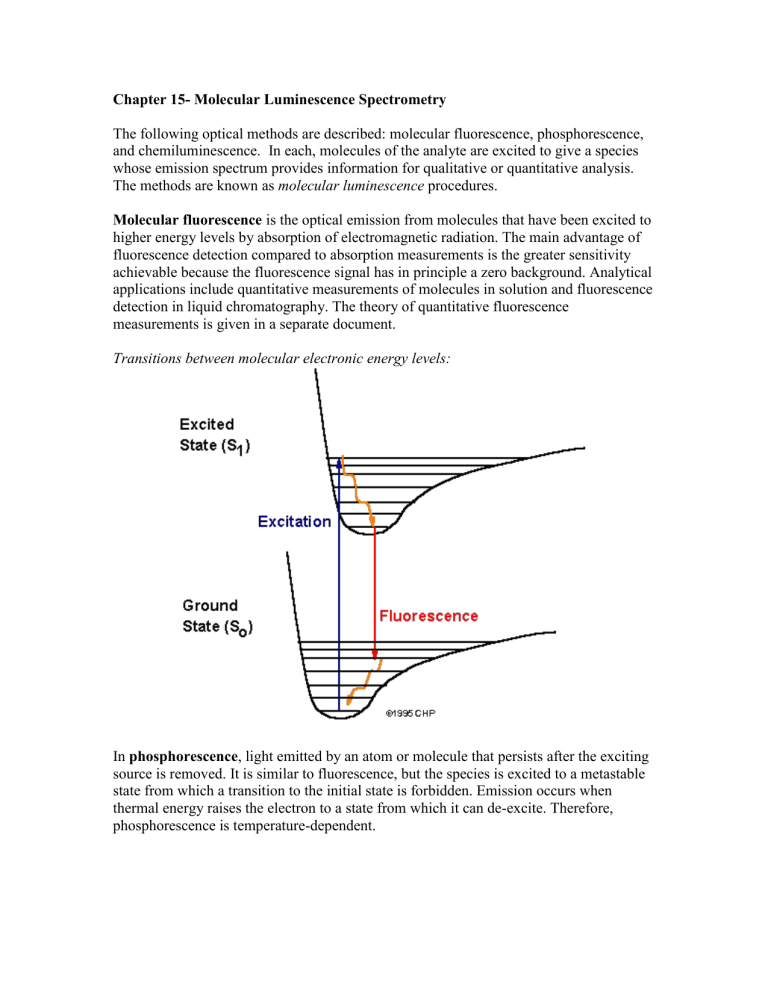
Molecular fluorescence is the optical emission from molecules that have been excited to higher energy levels by absorption of electromagnetic radiation. The main advantage of fluorescence detection compared to absorption measurements is the greater sensitivity achievable because the fluorescence signal has in principle a zero background. Analytical applications include quantitative measurements of molecules in solution and fluorescence detection in liquid chromatography. The theory of quantitative fluorescence measurements is given in a separate document.
Transitions between molecular electronic energy levels:
In phosphorescence , light emitted by an atom or molecule that persists after the exciting source is removed. It is similar to fluorescence, but the species is excited to a metastable state from which a transition to the initial state is forbidden. Emission occurs when thermal energy raises the electron to a state from which it can de-excite. Therefore, phosphorescence is temperature-dependent.
Reactions that produce light without heat are called chemiluminescent reactions.
Perhaps the most familiar chemiluminescent reactions are those that occur in living organisms. Fireflies produce light without heat by a chemiluminescent reaction.
To see the process of chemiluminescence, check out the movie below (provided by Sam
Houston State University- http://www.shsu.edu/~chm_tgc/chemilumdir/chemiluminescence2.html
)
Luminol Movie ( Quicktime ) ( AVI )
Fluorescence and phosphorescence are alike in that excitation is brought about by absorption of photons. As a consequence, the two phenomena are often referred to by the more general term photoluminescence.
Photoluminescence spectroscopy
Photoluminescence spectroscopy is a contactless, nondestructive method of probing the electronic structure of materials. Specifically, light is directed onto a sample, where it is absorbed and imparts excess energy into the material in a process called "photoexcitation." One way this excess energy can be dissipated by the sample is through the emission of light, or luminescence. In the case of photo-excitation, this luminescence is called photoluminescence. The intensity and spectral content of this photoluminescence is a direct measure of various important material properties.
More specifically, photo-excitation causes electrons within the material to move into permissible excited states. When these electrons return to their equilibrium states, the excess energy is released and may include the emission of light (a radiative process) or may not (a nonradiative process). The energy of the emitted light—or photoluminescence—is related to the difference in energy levels between the two electron states involved in the transition—that is, between the excited state and the equilibrium state. The quantity of the emitted light is related to the relative contribution of the radiative process.
Photoluminescense Applications:
Band gap determination . The most common radiative transition in semiconductors is between states in the conduction and valence bands, with the energy difference being known as the band gap. Band gap determination is particularly useful when working with new compound semiconductors.
Impurity levels and defect detection. Radiative transitions in semiconductors also involve localized defect levels. The photoluminescence energy associated with these levels can be used to identify specific defects, and the amount of photoluminescence can be used to determine their concentration.
Recombination mechanisms. As discussed above, the return to equilibrium, also known as "recombination," can involve both radiative and nonradiative processes.
The amount of photoluminescence and its dependence on the level of photoexcitation and temperature are directly related to the dominant recombination
process. Analysis of photoluminescence helps to understand the underlying physics of the recombination mechanism.
Material quality . In general, nonradiative processes are associated with localized defect levels, whose presence is detrimental to material quality and subsequent device performance. Thus, material quality can be measured by quantifying the amount of radiative recombination.
Special Features:
Various excitation wavelengths allow for varying penetration depths into the material, and thus, varying levels of volume excitation.
Detection of photoluminescence from 0.4 to 2.8 micrometers using diffraction and
Fourier-transform-based systems.
Mapping capabilities with 1-micrometer spatial resolution on the Fouriertransform-based system.
Sample temperatures of 4 to 300 K.
Sensitivity down to the level of parts per thousand, depending on impurity species and host.
Chemiluminescence is based upon the emission spectrum of an excited species that is formed in the course of a chemical reaction. In some instances, the excited species is the
product of a reaction between the analyte and a suitable reagent. The result is a spectrum characteristic of the oxidation product of the analyte.
Process of Excitation and Emission
•
Absorption of light -10
-15 s and related to
•
Vibrationalrelaxation
–excess vibrationalenergy in solution immediately lost in solution due to collisionaldeactivation, 10
-12 s.
•
Internal conversion
–intermolecular process by which a molecule passes to a lower energy electronic state without emission of light. Overlap of vibrationalenergy levels in two electronic energy levels.
•
External conversion
–deactivation of an excited electronic state by interaction and energy transfer between the excited molecule and solvent or other solutes.
•
Intersystem crossing
–process in which spin of an excited electron is reversed and change in multiplicity results. Most common when vibrationalmanifold overlap exists and when the molecule has a heavy atom substituent(e.g., Br, I).
•
Fluorescence and Phosphorescence– relaxation of an excited state via light emission. Time scales range from 10 -6 s to 100’s s.
A: Theory of Fluorescence and Phosphorescence:
Fluorescence occurs in simple as well as in complex gaseous, liquid, and solid chemical systems. Resonance radiation or resonance fluorescence is a type of fluorescence in which the absorbed radiation is remitted without a change in frequency. Molecular fluorescence (or phosphorescence) bands are found centered at wavelengths that are longer than the resonate line.
This shift toward longer wavelengths, or lower energies, is termed the
Stoke’s shift.
Resonance vs. non-Resonance Fluorescence-
Non-Radiant loses result in red shift in fluorescence.
Excitation at fixed wavelength and recording the emission spectra.
I: Excited States Producing Fluorescence and Phosphorescence:
The characteristic of fluorescence and phosphorescence spectra can be rationalized by means of the simple molecular orbital considerations. An understanding of the difference between the two photoluminescence phenomena requires a review of electron spin and single/triplet excited state.
Electron Spin
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This restriction requires that no more than two must have opposed spin states. Because of spin pairing, most molecules exhibit no net magnetic field and are thus said to be diamagnetic. In contrast, free radical, which contain unpaired electrons, have a magnetic moment are said to be paramagnetic.
Singlet/Triplet Excited States
A molecular electrons state in which all electron spins are paired is called a singlet state and no splitting of electronic energy levels occurs when the molecule is exposed to a magnetic field. The ground state for a free radical, on the other hand, is a doublet state because the odd electron can assume two orientations in a magnetic field, which imparts slightly different energies to the system.
The lowest heavy horizontal line represents the ground-state energy of the molecule, which is normally a singlet state and is labeled So. At room temperature, this state represents the energies of essentially al of the molecules in a solution.
II: Rates of Absorption and Emission:
The rate at which a photon of radiation is absorbed is enormous, the process requiring on the order o f 10
-14
to 10
-15 s. Fluorescence emission, on the other hand, occurs at a significantly slower rate. Here, the lifetime of the excited state is inversely related to the molar absorptivity of the absorption peak corresponding to the excitation process.
III: Deactivation Processes:
An excited molecule can return to its ground state by a combination of several mechanistic steps. Both processes involve the emission of a photon of radiation. The deactivation steps, indicated by wavy arrows, are radiationless processes. The favored route to the ground state is the one that minimizes the lifetime of the excited state. Thus, if deactivation by fluorescence is rapid with respect to the radiationless processed, such emission is observed. On the other hand, if a radiationless path has more favorable rate constant, fluorescence is either absent or less intense.
Vibration Relaxation
This relaxation process is so efficient that the average lifetime of a vibrationally excited molecule is 10-12s or less, a period significantly shorter than the average lifetime of an
electronically excited state. As a consequence, fluorescence form solution, when it occurs, always involves a transition from the lowest vibrational level of an excitation level of an excited state. A consequence of the efficiency of vibrational relaxation is that the fluorescence band for a give electronic transition is displaced toward lower frequencies or longer wavelengths from the absorption bands ( the stokes shift).
Internal Conversion
The term internal conversion describes intermolecular processes by which a molecule passes to a lower energy electronic state without emission of radiation. These processes are neither well defined nor well understood, but it is apparent that they are often highly efficient, because relatively few compounds exhibit fluorescence.
Internal conversion may also result in the phenomenon of predissociation.
Predissociation should be differentiated from dissociation, in which the absorbed radiation excites the electron of a chormophore directly to a sufficiently high vibrational level to cause rupture of the chromophoric bond; no internal conversion is involved.
Dissociation processes also compete with the fluorescence process.
External Conversion
Deactivation of an excited electronic state may involve interaction and energy transfer between the excited molecule and the solvent or other solutes. These processes are called collectively external conversion, or collisional quenching. Evidence for external conversion includes the marked effect upon fluorescence intensity exerted by the solvent; furthermore, those conditions that tend to reduce the number of collisions between particles generally lead to enhanced fluorescence.
Intersystem Crossing
Intersystem crossing is a process in which the spin of an excited electron is reserved, and a charge in multiplicity of the molecule results. As with internal conversion, the probability of this transition in enhanced if the vibrational levels of the two states overlap. Intersystem crossing in most common in molecules that contain heavy atoms, such as iodine or bromine. Spin/orbital interactions become large in the presence of such atoms, and a charge in spin is thus more favorable. The presence of paramagnetic species such as molecular oxygen in solution also enhances intersystem crossing and a consequent decrease in fluorescence.
Phosphorescence
Deactivation of electronic excited states may also involve phosphorescence. After intersystem crossing to an triplet state, further deactivation can occur either by internal or external conversion or by phosphorescence. External and internal conversions compete so successfully with phosphorescence that this kind of emission is ordinarily observed only at low temperatures, in highly viscous media or by molecules that are adsorbed on solid.
IV: Variables That Affect Fluorescence and Phosphorescence:
Both molecular structure and chemical environment influence whether a substance will or will not luminesce; these factors also determine the intensity of emission when
luminescence does occur. The effects of some of these variables are considered in this section.
Quantum Yield
The quantum yield, or quantum efficiency, for fluorescence or phosphorescence is simply the ratio of the number of molecules that luminesce to the total number of excited molecules. The quantum efficiency for highly fluorescent molecules approaches infinity, while the efficiencies for chemical that do not fluoresce approach zero.
Transition Types in Fluorescence
Fluorescence is seen with transitions from
*->
and
*->n processes. An electronically excited molecule ordinarily returns to its lowest excited state by a series of rapid vibrational relaxations and internal conversions that produce no emission or radiation.
Therefore, fluorescence is seen with a transition from the lowest vibrational level of the first excited electronic state to one of the vibrational levels of the electronic ground state.
For the majority of fluorescent compounds, then, radiation is produced by either an n->
* or a
->
* transition.
Quantum Efficiency and Transition Type
Fluorescence is more commonly associated with
->
* transitions because such transitions exhibit shorter average lifetimes and because the deactivation processes that compete with fluorescence are less likely to occur.
Fluorescence and Structure
The most intense and the most useful fluorescence is found in compounds containing aromatic functional groups with low-energy
->
* transition levels. Compounds containing aliphatic and alicyclic carbonyl structures or highly conjugated double-bond structures may also exhibit fluorescence, but the number of these is small compared with the number in the aromatic systems.
Effect of Concentration on Fluorescence Intensity
V: Emission and Excitation Spectra:
An excitation spectrum is obtained by measuring luminescence intensity at a fixed wavelength while the excitation wavelength is varied. Fluorescence and phosphorescence spectra involve excitation at a fixed wavelength while recording the emission intensity as a function of wavelength.
B: Instruments for Measuring Fluorescence and Phosphorescence:
The various components of instruments for measuring photoluminescence are similar to those found in ultraviolet/visible photometers or spectrophotometers. Nearly all fluorescence instruments employ double-beam optics as shown in order to compensate for fluctuations in the power of the source. The sample beam first passes through an excitation filter or a monochromator, which transmits radiation that will excite fluorescence but excludes or limits radiation of the wavelength of the fluorescence emission.
I: Components of Fluorometers and Spectrofluorometers:
Sources
A more intense source in needed than the tungsten of hydrogen lamp.
Lamps
The most common source for filter fluorometer is a low-pressure mercury vapor lamp equipped with a fused silica window. For spectrofluorometers, a 75 to 450-W highpressure xenon arc lamp in commonly employed.
Lasers
Most commercial spectrofluorometers utilize lamp sources because they are less expensive and less troublesome to use.
Filters and Monochromators
Both interface and absorption filters have been used in fluorometers for wavelength selection of both the excitation beam and the resulting fluorescence radiation. Most spectrofluorometers are equipped with at least one and sometimes two grating monochromators.
Transducers
Photomultiplier tubes are the most common transducers in sensitive fluorescence instruments.
Cell and Cell Compartments
Both cylindrical and rectangular cell fabricated of glass or silica are employed for fluorescence measurements.
II: Instrument Designs:
Fluorometers
Fluorometer schematic:
Spectrofluormeters
References: http://www.acs.org http://www.cas.org http://www.chemcenter/org http://www.sciencemag.org http://www.kerouac.pharm.uky.edu/asrg/wave/wavehp.html
http://www.chemistry.msu.edu/courses/cem333/Chapter%2015%20-
%20Molecular%20Luminescence%20Spectrometry.pdf http://elchem.kaist.ac.kr/vt/chem-ed/spec/molec/mol-fluo.htm http://www.shsu.edu/~chm_tgc/chemilumdir/chemiluminescence2.html