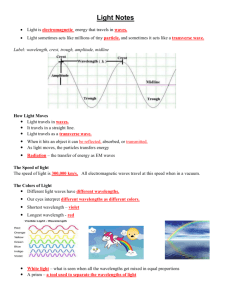
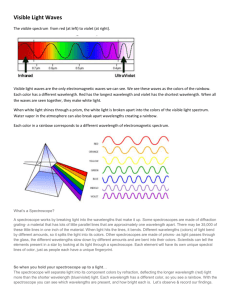
Light
advertisement

Light isn’t really a particle or a wave, but it has properties of both. Evidence that light is a wave: 1) Light waves undergo constructive and destructive interference. Lasers are and example of constructive interference. In the “double slit” experiment, light waves emanating from two adjacent slits overlap, forming bright spots (constructive interference) and dark spots (destructive interference). 2) Light waves can be polarized. The polarization of light indicates that is a transverse wave. 3) Light waves display Doppler shifts. Weather Radar, police radar guns, and astronomic measurements of the motion of stars, all take advantage of Doppler shifts of light waves. 4) Light waves refract (bend) when changing from one medium to another, just like water waves or sound waves. Other evidence indicates that light is made up of particles called photons. Photons have no mass, only energy equivalent to the frequency of light they represent. Evidence that light is a particle: 1) The slow exposure of photographic film shows a photon by photon development. 2) The photoelectric effect that allows solar cells to work cannot be explained by the wave behavior of light. Photons must have a certain threshold energy to knock electrons loose on the solar cell. When charged particles (such as electrons or ions) vibrate, some of their energy is converted into electromagnetic oscillations known as electromagnetic radiation (or light when it has a wavelength that is visible to the human eye). For the purposes of this class, we will refer to all electromagnetic radiation as light, regardless of its wavelength. Light is properly thought of as being both a wave and a particle. When light is thought of as a particle, the particles are called photons. Think of a photon as a bullet of energy that has no mass and travels at the speed of light. Certain phenomena associated with light can only be explained if light is made of particles. The photoelectric effect, which allows solar cells to work, and the photon-by-photon exposure of photographic film are two examples of light displaying particle behavior. We will focus instead on light’s many wave characteristics. All forms of light can be made to display the following wave characteristics: frequency/wavelength, amplitude, constructive and destructive interference, polarization, reflection, and refraction. Frequency Just like a tuning fork oscillating at a certain frequency produces sound waves of a similar frequency, electrons oscillating at a given frequency produce light waves of the same frequency. Example: KMDD broadcasts at a base frequency of 107.7 million Hz. This means that electrons in the radio tower are being forced to oscillate 107,700,000 times a second, which in turn produces light waves with the same frequency and a wavelength of 2.78 meters (which are too long for your eyes to detect, but which cause the electrons in the antenna of your radio to oscillate at 107.7 MHz) The human eye is only sensitive to wavelengths between 4 10-6 m and 7 10-6 m , which is just a small portion of the electromagnetic spectrum (the total range of wavelengths of light). Any time an atom absorbs energy, the energy is used to lift an electron up to a higher energy level. When the electron falls back down to a lower energy position, it produces a light wave of a specific wavelength — the more energy that was absorbed, the greater the energy that is released when the electron drops back down, and the shorter the wavelength of the light wave that is produced. One way for an atom to absorb energy is to crash into another one due to the random atomic movements that give a substance its temperature. When heated, the atoms in a substance crash together more violently, causing more energy to be absorbed by electrons in the atoms during the collisions, and therefore more energy to be released by the electrons after the collisions. This is why as a substance gets hotter it emits light of shorter wavelengths. The temperature of an object can be determined by the spectrum of light waves it produces. All objects have a temperature, and therefore all objects emit light waves. You glow in the dark, but the highest frequency light that you give off is only in the infrared portion of the electromagnetic spectrum. The wavelengths you give off are too long for your eyes to see, but are just right for a rattlesnake. The temperature of distant stars and even space itself can be determined by analyzing the spectrum of light emitted. Just like a radio can be tuned to certain frequencies, your eyes are tuned to certain frequencies. The retina of you eye contains specialized nerve cells that send an electric signal to the brain when they are stimulated. There are four types of light sensitive cells in the human retina. We have three types of cone cells, each of which is sensitive to light wave of a different range of wavelengths. These cone cells are what allow us to see color. Although light comes in many wavelengths, our eyes detect only three — red, green, and blue. Because our eyes are “tuned” to these three frequencies red, green, and blue are the three primary colors of light. (that’s right — yellow is not a primary color of light) Visible light of low frequency stimulates only the red-sensitive cone cells and we see red. Light in the middle range of the visible spectrum stimulates the green-sensitive cells and we see green. But if yellow light strikes the retina, it stimulates both the red and the green cells and we see yellow, even though we don’t have any yellow-sensitive cells. If all three types of cone cells (red, green, blue)are stimulated equally you see white. A color TV has only three colors that it can make (red, green, blue), but it can trick your brain into seeing all the colors of the spectrum by using different amounts of each of the three primary colors. There is a fourth type of light-sensitive cell in the retina called a rod cell that can be triggered by any wavelength of visible light. Rod cells see only light and dark, not color, but rod cells are much more sensitive to light than cone cells are. At night, when there isn’t enough light to trigger your cone cells, you can’t see color, just light and dark as perceived by you rod cells. Red, Green, and Blue are the three primary colors of light because these are the frequencies for which we have nerve cells to detect. If we had four types of color-sensitive nerve cells in our retinas, we would have four primary colors. Bees see ultraviolet frequencies, and some snakes see infrared frequencies. People who are color blind still see colors, they just have a hard time differentiating between colors because one type of cone cell (usually red or green) does not function properly. In a sense, color blind people have only two primary colors. The most common form of color blindness is red-green color blindness. Nature did not choose the three primary colors at random, they correspond to the low frequency (red), middle frequency (green), and high frequency (blue) parts of the visible spectrum. The visible spectrum is the total range of electromagnetic wavelengths that are visible to the human eye. Although there are millions of frequencies between the low end of the visible spectrum and the upper end, it is customary to divide it into seven colors: red, orange, yellow, green, Blue, indigo, and violet (ROY G BIV). Red has the lowest frequency and longest wavelength of visible light, blue has the highest frequency and shortest wavelength. Mixing colors If you shine a red light onto a screen, you see a red spot because only your red cone cells are activated by looking at the spot. If you shine a green light onto a screen, you see a green spot because only your green cone cells are activated by looking at the spot. But if you shine both a green light and a red light onto the same part of the screen, when you look at it, both your red cells and your green cells are activated, causing you to see a yellow spot, even if there are no yellow wavelengths present. When both your red and green cells are stimulated you see yellow. When both your blue and red cells are stimulated you see magenta.. When both your blue and green cells are stimulated you see cyan. When all three color cells (red, green, and blue) are stimulated equally, you see white. When none of your cone cells are stimulated you see black (if there is no light) or gray (if there is some light, but not enough to stimulate your cone cells). A pigment is an atom or molecule that absorbs some frequencies of light, but not others. The electrons that orbit and atom or molecule have a natural frequency, and when light hits at that frequency, resonance occurs causing the electron to vibrate enough that it sets the whole atom or molecule in motion. The energy at this frequency is transferred to the atom/molecule as heat, and is therefor not re-emitted. In this way a pigment subtracts out any light that hits it with a frequency equal to its natural frequency. A banana looks yellow because the pigment in the skin absorbs blue light and reflects red and green. White light shine on the banana, blue is absorbed and the reds and greens enter your eye and you see it as yellow. Q: What color would a banana look in red light? A: Red. It reflects both red and green, but if there is no green light shining on it, then it can not reflect green, only red. Q: What color would a banana look in green light? A: Green. It reflects both red and green, but if there is no red light shining on it, then it can not reflect red, only green. Q: What color would a banana look in blue light? A: Black. Yellow pigment is yellow because it absorbs blue light. If only blue light shines on the banana, then that light is absorbed, none is reflected and the banana looks black. A filter is a transparent substance that absorbs certain wavelengths. Example: A filter absorbs red frequencies of light. If white light passes through the filter, the red wavelengths are removed and the light that comes through looks cyan. red green green + blue = cyan blue If blue light was shined on the same filter, it would all pass through unabsorbed, and the light that emerged from the filter would still look blue — not cyan. Blue blue If yellow light was passed through the filter, the red wavelengths would be absorbed, and only the green wavelengths would get through. Red Green green