Solids, Glasses Lab
advertisement

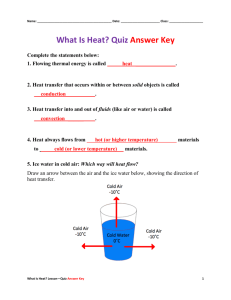
Solids, Glasses Lab. 1. A. Place one pellet of NaOH on a glass plate. Let it stand for a period or longer. Observe it occasionally. DO NOT TOUCH. Dispose of by washing down the drain. It’s a deliquescent material; what’s that mean? B. Place a tiny amount of blue CuSO4●5H2O crystals in a dry test tube. Heat, gently at first, until the color has gone. What has happened? What do you notice near the mouth of the test tube? Write an equation. II. A. Hold a thin strip of zinc metal with forceps. Heat the end strongly in the burner. Observe. Keep feeding the metal into the flame. B. Repeat, with thin copper wire. You will need your burner adjusted for maximum heat. Observe C. Repeat, using a piece of thin glass rod or tubing, a super cooled liquid rather than the crystalline solid metals used in A and B. Observe III. A. Cut off a 4-5 inch piece of glass tubing, by making ONE hard, deep scratch with a file. Quickly pick up the glass, face the scratch away from you, and place your two thumbs adjacent to the scratch on the opposite side of the tubing. Apply both bending and stretching force to the glass. And it will break cleanly at the scratch. WARNING: small piece of glass may flake off; wear goggles. Fire polish the very sharp broken end. Hold the end in the hottest part of the f lame, and slowly rotate it to get even heating of the sharp edge. It will quickly soften and flow into a smooth rounded edge. Excess heating will close up the hole. WARNING; Allow the glass to fully cool before it touches ANYTHING—especially skin. Rapid cooling by fanning, water, touching the lab bench, etc. will cause it to crack. Glass is a poor heat conductor; there may be only a cm. or so between places cool enough to hold and one hot enough to burn you. Feel for heat with the back of your hand (DON’T LET IT TOUCH YOUR SKIN), then touch gently before firmly grasping it. Bend/Right Angle: When the fire-polished ends are cool, hold the tube by the ends, and heat the middle in the burner. Rotate for even heating, and heat about 1.5 inches of glass. When sufficiently hot, the glass will start to sag. Guide it into a smooth right angle bend, without thinning of the tube. Wiggle the glass gently as it cools, to feel the stiffening of the glass as the viscosity increases with cooling. Show the bend to the teacher for approval. B. Stretch: Reheat the middle of the bend, getting it even hotter than before; you’ll have to rotate it to keep it from sagging. When as hot as possible, quickly REMOVE IT FROM THE FLAME and pull the ends apart to full arms’ length. Examine your “fiberglass”. Is it still hollow? C. Bubble: Take the longest piece of glass you have left (or cut a new one if you haven’t one about 4 inches long). Fire polish one end, cool, then heat the other end until it seals closed. Get the closed end as hot as possible, until it’s red hot and very soft and saggy. REMOVE FROM FLAME and very quickly blow in the open end as hard as you can. If done right, you’ll blow a bubble. Excessive blowing will bust the bubble, scattering bits of glass. WEAR GOGGLES. Show your teacher D. Stirring rod. Cut off 6-8 in. of solid glass rod (or end-sealed tubing). Fire polish one end to a smooth ball. Heat the other end, and flatten it with tongs or pressure against the bench to a spatula-like end. Show your teacher. Report all observations, answer questions, get teacher’s initialed approval for all glass work. Answer to Questions: 1. A. Deliquescent ? B. What happened to the CuSO4●5H2O ? Equation: CuSO4●5H2O + What appeared at the mouth of the test tube? q = Observations: I. A. NaOH left on open dish: II. A. What happened to the zinc strip? (did it melt at a specific temp?) B. What happened to the copper ? (did it melt at a specific temp?) C. What happened to the glass? (did it melt at a specific temp?) Teachers Initials for: Bend Stretch Bubble Stirring Rod