MKMoodley JR09-6434 supplementary information
advertisement

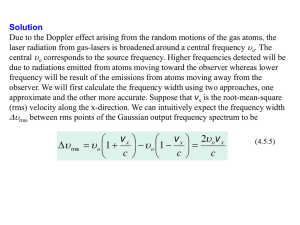
Supplementary information for “In-situ optical emission study on the role of C2 in the synthesis of single-walled carbon nanotubes” David Edmond Motaung National Centre for Nano-Structured Materials, Council for Scientific and Industrial Research, South Africa Mathew Kisten Moodleya) National Centre for Nano-Structured Materials, Council for Scientific and Industrial Research and School of Physics, University of the Witwatersrand E Manikandan National Centre for Nano-Structured Materials, Council for Scientific and Industrial Research Neil J Covilleb) School of Chemistry, University of the Witwatersrand S1.1 Target temperature calculations The rise in the target temperature when it absorbs a pulsed nanosecond laser can be estimated by :1 T T0 1 R E C pVHAZ (1.1) and VHAZ w2 t pulse (1.2) and K C p (1.3) VHAZ in eqn. 1.3 gives an estimate of the heat volume. The meaning of the symbols and their values are: w is the radius of the laser beam at the target surface in metres, ρ is the density of graphite in kg.m-3, Cp is the heat capacity, t is the laser pulse width in seconds. K is the thermal conductivity, E is the energy in joules, To is the initial temperature which is equal to the furnace temperature and R is the reflectivity of the target. The values for the density, heat capacity and thermal conductivity are those of graphite and were taken from www.azonano.com2. Therefore, using w= 1.5 x 10-3 m, ρ =2000 kg/m3, Cp=830 J/kg.K, t=15 x 10-9 s, K= 470 W/m.K, E= 1.07 J, T0= 1273 K, R=0.01. We get VHAZ = 1.5 x 10-11 m3. The temperature rise of the target is T= 4.508 x104 K or about 45 000 K as indicated in the main article. The laser energy is absorbed within 15 ns. So the heating rate would be 3 x 1012 K/s. We estimate that the volume of material ejected in a one laser shot equals VHAZ and from this we deduce for carbon, this equates to about 1.5 x1018 atoms. Hence our estimate is 1018-1019 atoms as stated in the main article. S1.2 Plasma expansion velocity We use the following equation to estimate the plasma expansion velocity in m.s-1 :3 v 2 kT (1.4) M Where η is the number of internal degrees of freedom and can be between 2.53 and 3.28. We use η =2.5. M is the mass of the species. T is the temperature in Kelvin and k is the Boltzmann constant. For a single carbon atom, at T= 10000 K, v= 5.886 x 103 m.s-1≈5.9 km.s-1. S1.3 Why do we neglect the effect of Doppler broadening? The effect of Doppler broadening on the emission lines of CII was neglected. Doppler broadening is estimated from:4 a Corresponding authors a) MKMoodley@csir.co.za and b) Neil.Coville@wits.ac.za D V c (1.5) Where c is the speed of light and is the emission line of an atom or ion. In our case we choose CII at 283.7 nm. Using the velocities obtained from eqn. 1.4, we find that is = 5.566 x 10-12 m. The minimum resolving limit of the Andor spectrograph is: 5.104 (1.6) Using the emission line of CII ion at 283.7 nm, we find that = 5.674 x 10-12 m, which is slightly larger than D . Therefore the effect of Doppler broadening would be not be able to be resolved with the Andor ME5000 spectrograph used in this study. S1.4 XRD of the target XRD measurements were made on the target used in this study. A Panalytical X’pert PRO PW 3040/60 x-ray diffractometer with a Cu K ( = 0.154 nm) monochromated radiation source, operating at 45.0 kV and 40.0 mA were employed to characterize the materials. XRD data were collected in the 2 ranging from 10 to 100 with a step size of 0.02. The target was already subjected to a temperature of 1273 K in argon gas. It was allowed to cool to room temperature. XRD pattern of Figure suppXRD identifies the presence of Ni3C [JCDPS file: 00-06-0697], graphite [JCDPS file: 00-01-0646], YC2 [JCDPS file: 00-82-0628] and Y2O3 [JCDPS file: 00-41-1105]. Yttrium may have oxidized at room temperature. No oxides of nickel were detected. Figure S1. XRD pattern of the target used for the synthesis of SWCNTs. 1 C. P. Grigoropoulos, in Laser Ablation and Desorption, 1stth edition, edited by J. C. Miller (Academic Press, San Diego, 1998), Vol. 30, Chap. 4, p.173. 2 http://www.azom.com/details.asp?articleid=1630 S.S. Harilal, R.C. Isaac, C.V. Bindhu, V.P.N. Nampoori, and C.P.G. Vallabhan, J. Appl. Phys. 81, 3637 (1997). 3 S. S. Harilal, R. C. Isaac, C. V. Bindhu, V. P.N. Nampoori, and C.P.G. Vallabhan, J. Appl. Phys. 81, 3637 (1996). 4 Andor ME 5000 Echelle Spectrograph Operating Manual (2007)