Poster title - Department of Chemistry
advertisement
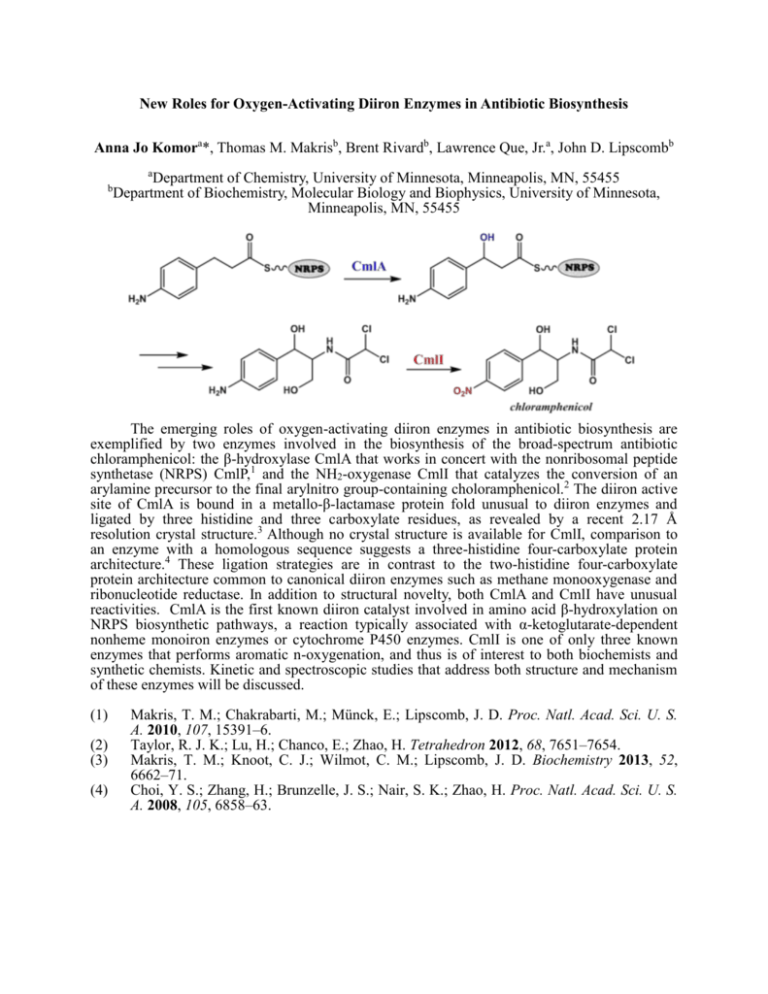
New Roles for Oxygen-Activating Diiron Enzymes in Antibiotic Biosynthesis Anna Jo Komora*, Thomas M. Makrisb, Brent Rivardb, Lawrence Que, Jr.a, John D. Lipscombb a b Department of Chemistry, University of Minnesota, Minneapolis, MN, 55455 Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota, Minneapolis, MN, 55455 The emerging roles of oxygen-activating diiron enzymes in antibiotic biosynthesis are exemplified by two enzymes involved in the biosynthesis of the broad-spectrum antibiotic chloramphenicol: the β-hydroxylase CmlA that works in concert with the nonribosomal peptide synthetase (NRPS) CmlP,1 and the NH2-oxygenase CmlI that catalyzes the conversion of an arylamine precursor to the final arylnitro group-containing choloramphenicol.2 The diiron active site of CmlA is bound in a metallo-β-lactamase protein fold unusual to diiron enzymes and ligated by three histidine and three carboxylate residues, as revealed by a recent 2.17 Å resolution crystal structure.3 Although no crystal structure is available for CmlI, comparison to an enzyme with a homologous sequence suggests a three-histidine four-carboxylate protein architecture.4 These ligation strategies are in contrast to the two-histidine four-carboxylate protein architecture common to canonical diiron enzymes such as methane monooxygenase and ribonucleotide reductase. In addition to structural novelty, both CmlA and CmlI have unusual reactivities. CmlA is the first known diiron catalyst involved in amino acid β-hydroxylation on NRPS biosynthetic pathways, a reaction typically associated with α-ketoglutarate-dependent nonheme monoiron enzymes or cytochrome P450 enzymes. CmlI is one of only three known enzymes that performs aromatic n-oxygenation, and thus is of interest to both biochemists and synthetic chemists. Kinetic and spectroscopic studies that address both structure and mechanism of these enzymes will be discussed. (1) (2) (3) (4) Makris, T. M.; Chakrabarti, M.; Münck, E.; Lipscomb, J. D. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 15391–6. Taylor, R. J. K.; Lu, H.; Chanco, E.; Zhao, H. Tetrahedron 2012, 68, 7651–7654. Makris, T. M.; Knoot, C. J.; Wilmot, C. M.; Lipscomb, J. D. Biochemistry 2013, 52, 6662–71. Choi, Y. S.; Zhang, H.; Brunzelle, J. S.; Nair, S. K.; Zhao, H. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 6858–63.











