SUPPLEMENTAL METHODS AND DATA
advertisement

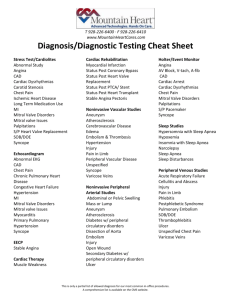
SUPPLEMENTAL METHODS AND DATA Supplemental Methods: Echocardiography: The mitral annular hinge points were traced, and mid-systolic tethering distances calculated from each papillary muscle tip to the opposite fibrous trigone [1, 2] along with PM tip separation (Supplemental Figure 4). The 3D Echo MV surface area quantification with Omni4D (MD Handschumacher, Boston, MA) has been validated against excised MV specimens in 15 sheep with excellent correlation and agreement (R2=0.86, SEE=1.24, mean difference=0.51±1.15, p=0.71) [3], and in vivo against CT in 14 patients (average difference 4.8±4.4% of mean, R2=0.96, intra- and interobserver variation coefficients of 4.2 and 4.4%) [4]. Molecular histopathology: MV leaflet and chordal cryosections (6µm) were stained for collagen composition using Masson trichrome method. To detect collagen architecture, leaflets were stained with picrosirius red visualized under circular polarized light (Eclipse 50i). Elastin was detected using the van Gieson method. Immunohistochemistry was performed with the avidin-biotin-peroxidase method previously described.[5] Valvular endothelial cells (VECs) were identified using mouse anti-sheep CD31 antibody (Santa Cruz Biotechnology). The activated valvular interstitial cell (VIC) phenotype was determined by α-smooth muscle actin antibody ([anti-α-SMA]; clone 1A4; Sigma) [5, 6]. Vascular cell adhesion molecule-1 (VCAM-1; DAKO) staining was used to assess endothelial-cell activation. CD45, considered a pan-hematopoietic marker, was identified by mouse anti-sheep CD45 (AbD Serotec); anti-Ki67 antibody (Abcam) determined cell proliferation. To explore mechanisms of cellular changes, sections were 1 immunostained with anti-TGF-β1 (R&D Systems) for latent and activated protein [7]. Extracellular matrix (ECM) turnover was assessed by immunostaining for matrix metalloproteinases (anti-MMP-2 and anti-MMP-9; Santa Cruz). Cell analysis. MV leaflet tissue was minced into 2-mm3 pieces and incubated with 3 ml of Cell Dissociation Buffer (Invitrogen), an enzyme-free, EDTA-containing solution for 4minutes at 37 C. Repeated pipetting produced a single-cell suspension. Flow-cytometry quantified cells transitioning between endothelial and mesenchymal phenotypes. VECs were quantified using murine anti-sheep CD31 antibody conjugated to fluorescein isothyocyanate (FITC, AbD Serotec); endothelial cells transitioning to a mesenchymal phenotype (EMT) were detected using murine anti-α-SMA conjugated to phycoerythrin (R&D Systems). Supplemental Results: While MI increased LV volumes relative to tethering-alone, adding LV constraint to the tethered+MI model yielded LV volumes comparable to those with tethering alone (Supplemental Figure 4; LV end-diastolic volume: 58±12 vs 63±13ml, p=0.529; LV endsystolic volume: 34±8 vs 31±8, p=0.566). 3D Echo analyses showed that tethered+MI sheep had increased tethering distances at sacrifice compared with tethered-alone sheep, but adding LV constraint in that model reduced tethering distances to values comparable to those with tethering-alone (Supplemental Figure 4). LV constraint therefore allowed us to compare MV adaptation in the setting of ischemia and MI with LV remodeling (tethered+MI group) versus limited LV remodeling (tethered+MI LV 2 constraint group). MV tenting volume was comparable in the tethered+MI+LV constraint and tethered-alone groups (2.11±0.54 vs 1.88±0.65 cm3, p=0.674). Supplemental Figures: Supplemental Figure 1. Progressively increased proportion of CD31+ cells that are also -SMA+ (upper right quadrant), indicating EMT, in tethered-alone and tethered+MI valves. Left, Representative sample of cells double-labeled with isotype-matched control antibodies. Right, Cells double-labeled with anti-sheep CD31 conjugated to fluorescein isothyocyanate and anti--SMA conjugated to phycoerythrin (PE). Compensation was performed with singly labeled cells (not shown). 3 Supplemental Figure 2. Heterogeneous collagen deposition and degradation in tethered+MI valves (left: Hematoxylin&Eosin stain; right: Masson’s trichrome). (Arrows: areas of dense subatrial collagen) Supplemental Figure 3. Compared to control there is elastin fiber degradation in tethered-alone and tethered+MI valves (Arrows: elastin staining black; Van Gieson stain). Note loss of elastin in tethered+MI valves. 4 Supplemental Figure 4. Left: Left ventricular end-diastolic and end-systolic volumes (LVEDV, LVESV) at sacrifice in tethered-alone vs tethered+MI vs tethered+MI LV constraint sheep. Note the limited LV remodeling in the tethered+MI LV constraint vs the tethered+MI sheep. Right: Schematic depicting the LV-papillary muscle-mitral valve tethering distances changes in tethered-alone vs tethered+MI vs tethered+MI LV constraint sheep. The double-arrowed red lines indicate a significant increase in interpapillary muscle (LP<->MP) and medial papillary muscle (MP)-to-lateral trigone (LT) distances only in the tethered+MI sheep (Distance between medial and lateral PM tips = 26±4mm with tethering+MI vs 21±3mm with tethering alone and 21±2mm with tethering+MI+LV constraint, p<0.05; distance from medial PM tip to lateral trigone = 40±3mm with tethering+MI vs 37±1mm with tethering-alone and 37±4mm with 5 tethering+MI+LV constraint, p>0.05). (AV=aortic valve; LV=left ventricle; LP=lateral papillary muscle; LT=lateral trigone; MP=medial papillary muscle; MT=medial trigone, MV=mitral valve) Supplemental References 1. Otsuji, Y., M.D. Handschumacher, E. Schwammenthal, L. Jiang, J.K. Song, J.L. Guerrero, G.J. Vlahakes, and R.A. Levine, Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation, 1997. 96(6): p. 1999-2008. 2. Kumanohoso, T., Y. Otsuji, S. Yoshifuku, K. Matsukida, C. Koriyama, A. Kisanuki, S. Minagoe, R.A. Levine, and C. Tei, Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. The Journal of thoracic and cardiovascular surgery, 2003. 125(1): p. 135-43. 3. Chaput, M., M.D. Handschumacher, F. Tournoux, L. Hua, J.L. Guerrero, G.J. Vlahakes, and R.A. Levine, Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation, 2008. 118(8): p. 845-52. 4. Beaudoin, J., W.E. Thai, B. Wai, M.D. Handschumacher, R.A. Levine, and Q.A. Truong, Assessment of mitral valve adaptation with gated cardiac computed tomography: validation with three-dimensional echocardiography and mechanistic insight to functional mitral regurgitation. Circulation. Cardiovascular imaging, 2013. 6(5): p. 784-9. 5. Aikawa, E., P. Whittaker, M. Farber, K. Mendelson, R.F. Padera, M. Aikawa, and F.J. Schoen, Human semilunar cardiac valve remodeling by activated cells from 6 fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation, 2006. 113(10): p. 1344-52. 6. Rabkin, E., M. Aikawa, J.R. Stone, Y. Fukumoto, P. Libby, and F.J. Schoen, Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation, 2001. 104(21): p. 2525-32. 7. Liu, A.C., V.R. Joag, and A.I. Gotlieb, The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol, 2007. 171(5): p. 1407-18. 7