Experiment QA1 Qualitative Analysis
advertisement
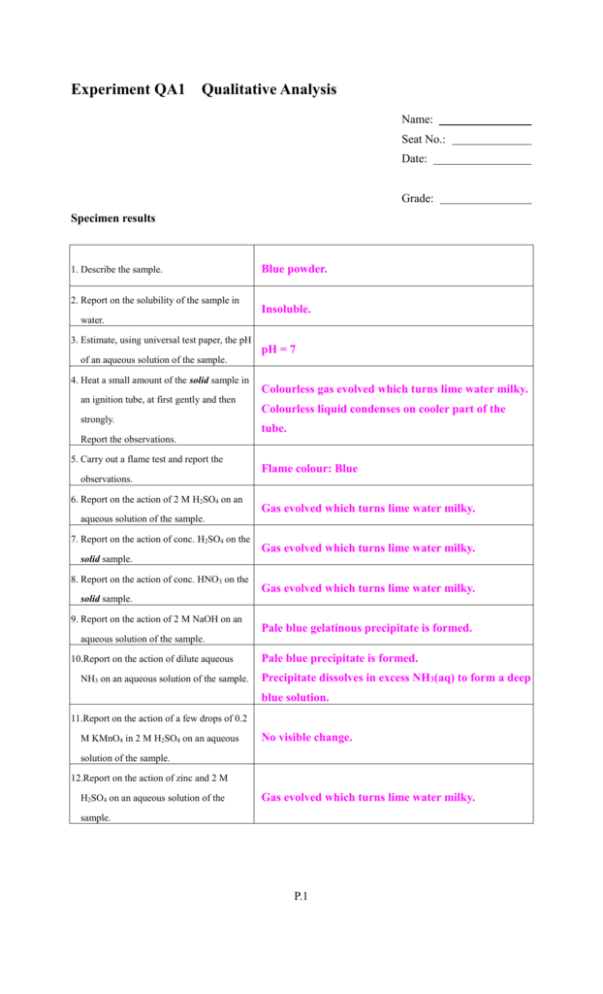
Experiment QA1 Qualitative Analysis Name: Seat No.: Date: Grade: Specimen results 1. Describe the sample. 2. Report on the solubility of the sample in Blue powder. Insoluble. water. 3. Estimate, using universal test paper, the pH pH = 7 of an aqueous solution of the sample. 4. Heat a small amount of the solid sample in an ignition tube, at first gently and then strongly. Colourless gas evolved which turns lime water milky. Colourless liquid condenses on cooler part of the tube. Report the observations. 5. Carry out a flame test and report the Flame colour: Blue observations. 6. Report on the action of 2 M H2SO4 on an Gas evolved which turns lime water milky. aqueous solution of the sample. 7. Report on the action of conc. H2SO4 on the Gas evolved which turns lime water milky. solid sample. 8. Report on the action of conc. HNO3 on the Gas evolved which turns lime water milky. solid sample. 9. Report on the action of 2 M NaOH on an Pale blue gelatinous precipitate is formed. aqueous solution of the sample. 10.Report on the action of dilute aqueous NH3 on an aqueous solution of the sample. Pale blue precipitate is formed. Precipitate dissolves in excess NH3(aq) to form a deep blue solution. 11.Report on the action of a few drops of 0.2 M KMnO4 in 2 M H2SO4 on an aqueous No visible change. solution of the sample. 12.Report on the action of zinc and 2 M H2SO4 on an aqueous solution of the Gas evolved which turns lime water milky. sample. P.1 From the result of the above tests, deduce which cation(s) and anion(s) may be present in the solid sample. The sample is a blue powder which is insoluble in water, it is neutral, its flame colour is blue (inference: Cu2+), when the sample is heated strongly, a colourless gas is evolved which turns lime water milky (inference: CO32-, HCO3-), and a colourless liquid condenses on the cooler part of the ignition tube (inference: H2O). An aqueous solution of the sample reacts with dil. and conc. acid with evolution of a colourless gas which turns line water milky (inference: CO32-, HCO3-). When an aqueous solution of the sample is treated with dil. NaOH, a pale blue gelatinous precipitate is formed. When treated with NH3(aq), a pale blue gelatinous precipitate is formed first and the precipitate dissolves in excess NH3(aq) to form a deep blue solution (inference: Cu2+). Therefore, the sample is a hydrated salt, it may contains Cu2+ ions and CO32- ions. P.2









