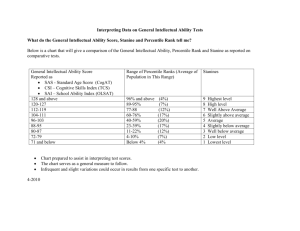
Books, Drugs and Seeds: the Politics of Access
advertisement

Books, Drugs and Seeds: the Politics of Access Susan K. Sell Professor of Political Science and International Affairs George Washington University Comments welcome: susan.sell@gmail.com Prepared for the Transatlantic Consumer Dialogue, “The Politics and Ideology of Intellectual Property”, March 20-21, 2006, Brussels; and the International Studies Association annual meeting, San Diego, March 22-26, 2006. Books, Drugs and Seeds: the Politics of Access1 Abstract In recent years developing countries, NGO activists, multinational corporations and their home countries increasingly have clashed over intellectual property policies. All intellectual property is not alike; for example, one could argue that movies and compact discs are not core components of economic development. One could not make that case for books, drugs and seeds. Access to educational materials (including scientific and other scholarly journals and educational software), life-saving medicines, and seeds for sustainable agriculture is the sine qua non of economic development. Education, health and food security are central components of economic development, yet the strong trend toward transforming these into private commodities for sale at premium prices through higher levels of intellectual property protection has made them less available to those who need them most. This paper examines the “North-South” politics of access to these essential inputs by analyzing the politics of multilateral and bilateral negotiations surrounding patent and copyright policies pertaining to books, drugs, and seeds. In particular, it identifies the structural features, the key actors and coalitions in each sector, and the multilateral institutions and bilateral treaties shaping the politics of access. It compares progress in each of these “access campaigns” and speculates about possible strategies that developing countries might use to increase access to books, drugs and seeds. Earlier versions of pieces of this argument have appeared as: Susan K. Sell, 2004. “The Quest for Global Governance in Intellectual Property and Public Health: Structural, Discursive, and Institutional Dimensions” Temple Law Review 77:2: 363-399; and Susan K. Sell, 2004. “What Role for Humanitarian Intellectual Property? The Globalization of Intellectual Property Rights” Minnesota Journal of Law, Science & Technology 6:1: 191-211. 1 Acronyms ABIA AIDS BIO CBD CGIAR COP CPTech DMCA DNA DRM EC EU FAO FTAA GATT GURTs HAI HIV IATP IGC IIPA IIPI IMF IP IPR IPC IUPGR MSF NAMA NGO NIH NIEO OAPI OECD PBRs PCT PGR PhRMA PTO SPLT TK TRIPS UNCTAD American BioIndustry Alliance acquired immunodeficiency syndrome Biotechnology Industry Organization Convention on Bio-Diversity Consultative Group on International Agricultural Research Conference of the Parties (CBD) Consumer Project on Technology Digital Millennium Copyright Act deoxyribonucleic acid digital rights management European Community European Union Food and Agriculture Organization Free Trade of the Americas Agreement General Agreement on Tariffs and Trade genetic use restriction technologies Health Action International human immunodeficiency virus Institute for Agriculture and Trade Policy Intergovernmental Committee on Intellectual Property, Genetic Resources, Traditional Knowledge and Folklore (WIPO) International Intellectual Property Alliance International Intellectual Property Institute International Monetary Fund intellectual property intellectual property right Intellectual Property Committee International Undertaking on Plant Genetic Resources (FAO) Medecins sans Frontieres Non-agricultural market access non-governmental organization National Institutes of Health (US) New International Economic Order Organisation Africaine de la Propriete Intellectuelle Organization for Economic Cooperation and Development Plant breeders’ rights Patent Cooperation Treaty plant genetic resources Pharmaceutical Research and Manufacturers Association Patent and Trademark Office (US) Substantive Patent Law Treaty Traditional Knowledge Agreement on Trade-Related Aspects of Intellectual Property Rights United Nations Conference on Trade and Development 4 UNDP UNESCO UNHCHR UNICEF UPOV USDA USTR WHA WHO WIPO WTO WWF United Nations Development Program United Nations Educational, Scientific and Cultural Organization United Nations High Commissioner on Human Rights United Nations Children’s Fund Union for the Protection of New Varieties of Plants United States Department of Agriculture United States Trade Representative World Health Assembly World Health Organization World Intellectual Property Organization World Trade Organization World Wildlife Fund 5 INTRODUCTION In recent years developing countries, NGO activists, multinational corporations and their home governments increasingly have clashed over intellectual property policies. All intellectual property is not alike; for example, one could argue that movies and compact discs are not core components of economic development. One could not make that case for books, drugs and seeds. Access to educational materials (including scientific and other scholarly journals and educational software), life-saving medicines, and seeds for sustainable agriculture is the sine qua non of economic development. While education, health and food security are central components of economic development, the strong trend toward transforming these into private commodities for sale at premium prices through higher levels of intellectual property protection has made them less available to those who need them most. This paper examines the “North-South” politics of access to these essential inputs by analyzing the politics of multilateral and bilateral negotiations surrounding patent and copyright policies pertaining to books, drugs, and seeds. In particular, it identifies the structural features, the key actors and coalitions in each sector, and the multilateral institutions and bilateral treaties shaping the politics of access. It compares progress in each of these “access campaigns” and speculates about possible strategies that developing countries might use to increase access to books, drugs and seeds. The dramatic expansion of intellectual property rights threatens to reduce access to life-saving medicines, essential crops and educational materials. For example, intellectual property policies have contributed to the high cost of essential medicines, keeping them out of reach of the world’s poor. Similarly, while advances in biotechnology may potentially enhance the nutritional content of basic crops (i.e., vitamin A-enriched "golden rice")2, regulatory trends and new technologies threaten to reduce access to such innovations for those who need it most. While the age of the Internet has increased potential access to educational materials, the digital divide notwithstanding, digital rights management technologies have hampered access to such materials. “The institution of property is extremely complex, and more importantly, political” (Aoki, 2003: 317). Yet we are no closer to resolving these controversies. “More often than not, rather than being an answer, the issue of property rights is only the beginning of a long series of vexing questions” (Coombe, 1998: 59). This paper begins by outlining what is at stake in these debates. It goes on to 2 Leaving aside the highly contentious issue of genetically modified organisms 6 discuss parallels between books, drugs and seeds and highlights how intellectual property protection can be an obstacle to access. The next section surveys the structural, discursive and institutional dimensions of the politics of access. The section on institutions addresses the debate over the role of the World Trade Organization (WTO) in relation to other international organizations. It then surveys intellectual property policymaking in the World Intellectual Property Organization (WIPO), the United Nations Food and Agriculture Organization (FAO), the Convention on Biological Diversity (CBD), the United Nations High Commissioner on Human Rights (UNHCHR) and the World Health Organization (WHO). The paper then concludes by addressing both obstacles to and opportunities for increasing access to books, drugs and seeds. WHAT IS AT STAKE? At the global level, the most important international public law governing intellectual property rights is the 1995 Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) administered by the World Trade Organization (WTO). Unlike most international law, TRIPS is binding and enforceable. The WTO may authorize states to sanction those found to be in violation of the agreement. TRIPS reflects the interests of intellectual property owners. Indeed, its very existence and much of its substance owe much to just a handful of global firms based in the United States (Braithwaite and Drahos, 2000: 12; Matthews, 2002: 7; Sell, 2003: 75). TRIPS extends patent rights for twenty years, requires developing countries to offer patent protection for pharmaceuticals, sharply circumscribes the conditions under which states may issue compulsory licenses, and reduces states’ autonomy in crafting domestic intellectual property policies that suit their diverse levels of innovation and economic development. From the standpoint of economic development and technology transfer, TRIPS represents the most challenging public international law. In a sharply worded and bold critique, a United Nations Development Program (UNDP) report stated that “‘[c]ountries at low levels of human technological capability cannot benefit significantly from TRIPS . . . . Developing countries are not likely to be even at least as well off under TRIPS as they would be outside it’” (McDowell, 2003). While some critics, such as the UNDP, call for TRIPS’s abolition, others argue that it is workable for developing countries if interpreted appropriately (Reichman and Lange, 1998: 49-68). Everyone agrees that the short-term consequences will be massive resource transfers from developing countries to owners of intellectual property. The World Bank has estimated that TRIPS should yield an annual nineteen billion dollars for the United States, whereas South Korea would sustain the largest loss – fifteen billion dollars (Newfarmer et al. 2001:137.) Countries that consume and import intellectual property will pay a higher premium to those who produce and export it. Under TRIPS, countries have agreed that importation of a product constitutes “working” the patent. However, importation represents only a passive mode of technology transfer and once again raises concerns that firms will use patents to maintain import monopolies. Those concerns had animated the earlier New International Economic Order (NIEO) approach to patents (Sell, 1998: 28-29). Another 7 consequence of TRIPS is that it offers “hardly any incentive for the patentee to license his technology. The technology holder can serve the large and small markets with his enhanced rights without licensing the technology” (Verma, S.K., 2001: 344). Overall, TRIPS reflects and promotes the interests of global corporations that seek to extend their control over their intellectual property. These firms, acting through the United States government (and with the support of Europe and Japan), largely captured the WTO process and succeeded in making public international law to suit their particular needs. The rationale for intellectual property rights is that they provide incentives for creation and the dissemination of innovation. Without the compensation made possible by intellectual property rights, public goods will be underprovided. However, the merits of granting exclusive rights to intellectual property owners have to be balanced against the economic effects of higher product and transaction costs and the potential "exclusion from the market of competitors who may be able to imitate or adapt the invention in such a way that its social value is increased" (Trebilcock and Howse, 1995: 250). In short, intellectual property rights reflect an inherent tension between creation and diffusion. Should intellectual property rights be treated as "a public goods problem for which the remedy is commodification, or a monopoly of information problem for which the remedy is unfettered competition" (Boyle, 1992: 1450)? The market-based, or commodification, justification for strong intellectual property rights is that patents and licenses provide incentives to "increase the number of commercially available products and thereby serve the public interest" (Lieberwitz, 2004: 782). However, it is important to ask which publics are served? In agriculture, stakeholders include private sector seed companies, public corporations, research institutes, and resource-poor farmers (UNCTAD-ICTSD, 2003: 108). In health, stakeholders include non-generic pharmaceutical companies, generic pharmaceutical companies, public sector health providers, and people who need health care. Rights-holders benefit, as do those who have the resources to participate in the commercial market. But market-based solutions alone fail to serve the poor and the marginalized, such as the millions afflicted with HIV/AIDS in Africa and Asia and smallholder subsistence farmers in much of the global South. Of the estimated 42 million infected with HIV/AIDS in the developing world, and the 6 million with full-blown AIDS who need anti-retroviral treatment to stay alive, only 300,000 are receiving these drugs and 100,000 of them are in Brazil (Lamptey, 2003: 650). Lettington observes that "approximately 75% of the world's undernourished are smallholder farmers" (Lettington, 2003: 7). Furthermore, about 75% of sub-Saharan Africa’s people are rural and depend upon agriculture for their livelihoods (Taylor and Cayford, 2004: 280). Market mechanisms to deliver innovation into the public domain fail spectacularly in the oligopolistic markets of the contemporary life sciences industries. Indeed, "international markets for technologies are inherently subject to failure due to distortions attributable to concerns about appropriability, problems of valuing information by buyers and sellers, and market power, all strong justifications for public intervention at both the domestic and global levels" (Maskus and Reichman, 2004: 288). Therefore, the policy 8 challenge is where to strike the balance, and to explore options that may maximize the benefits provided by intellectual property rights while minimizing the harms produced by over-extension of such rights. In this regard, policymakers must make room for humanitarian intellectual property policies that promote social goals such as protecting public health, alleviating malnutrition, and promoting the development of human capital though access to knowledge and educational materials. WHAT DO BOOKS, DRUGS AND SEEDS HAVE IN COMMON? What do books, drugs and seeds have in common? First of all, they are crucial to life chances. Furthermore, access to these items has the potential to spur lasting development and build capacity for innovation. For example, the World Health Organization (WHO) has recognized this through its list of essential medicines that should be available for public procurement (WHO), and the Food and Agriculture Organization (FAO) has maintained a list of 35 staple crops that no one may own in order to facilitate access. As Dutfield suggests, “to argue that imitation is an essential stage in learning to innovate and – that it can even be creative in itself – is stating the obvious. Admittedly, anybody with the right equipment can copy a music CD and will learn very little by doing it. But copying a new medicine, especially a complex protein-based drug, is another story entirely” (2005a). Secondly, each of these sectors is characterized by marked economic concentration that has only increased over the past several decades. The combination of expanded intellectual property rights and relaxed anti-trust enforcement has led to economic concentration in the life sciences industries. This situation has translated “economic power into greater influence over policymaking that has hitherto been seen as the realm of the public sphere” (Buse et. al., 2002: 261). In pharmaceuticals just since 1999, Zeneca acquired Astra, Hoescht acquired Marion Merrel Dow, Sandoz and Ciba-Geigy merged, Glaxo Wellcome and SmithKline Beecham merged, Pharmacia and Upjohn merged with Monsanto, Sanofi-Syntelabo SA was the object of a hostile takeover by Aventis, and Pfizer’s acquisitions made it the largest world company with revenues of $53 billion in 2004 (roughly 40% more than #2 GlaxoSmithKline) (Rosenberg, 2006: 65). The global market shares of the largest non-generic pharmaceutical companies in 2003 were as follows: Pfizer, 11%; GlaxoSmithKline, 6.9%; Merck & Co. 5%; AstraZeneca, 4.8%. and Johnson & Johnson, 4.7% (Rosenberg, 2006: 69). The vertical integration of plant breeding, agrichemical and food processing corporations led to a situation by the late 1990s in which "the top ten seed companies . . . control[led] 30 percent of the world's US$23 billion commercial seed market" (GAIA/GRAIN, 1998a: 13). Corporate plant breeders are obtaining broad patents that will have far reaching consequences. Breeders are patenting entire species (cotton), economic characteristics (oil quality), plant reproductive behavior (apomixis) and basic techniques of biotechnology (gene transfer tools) (GAIA/GRAIN, 1998a: 13). Six major industrial groups now control most of the technology "'which gives freedom to undertake commercial R&D in the area of GM crops". These are (i) Agrevo and Plant Genetic Systems (PGS); (ii) Du Pont and Pioneer; (iii) ELM, DNAP, Asgrow and Seminis; (iv) Monsanto, Calgene, DeKalb, Agracetus, PBI, 9 Hybritech and Delta and Pine Land Co.; (v) Novartis; and (vi) Zeneca, Mogen and Avanta" (Dutfield, 2003a: 170; see also Pistorius and van Wijk, 1998: 119-124). Furthermore, in agro biotechnology six companies alone hold 75% of all US patents granted to the top 30 patent-holding firms: Monsanto, Du Pont, Syngenta, Dow, Aventis, and Grupo Pulsar (Dutfield, 2003a: 154; see also Fowler, 1994: 146). Two multinational companies, Archer Daniels Midland and Cargill control about 75% of the global cereals trade; while 50% of the world’s coffee supply comes from smallholder farmers, 40% of global coffee trade is controlled by just four companies (IATP, 2005: 3). “High levels of corporate concentration mean most of the benefits of trade liberalization will be captured by multinational food giants such as Cargill, Nestle and Wal-Mart rather than by farmers and exporters in developing countries” (ActionAID, 2006: 12). This combination of economic concentration with extensive and broad patenting means that a handful of global corporations are making huge inroads toward control of the world's food supply and are entangling farmers an increasingly complex web of licensing and royalty obligations. Large mergers also have been the rule in the academic publishing industry, again leading to further economic concentration. Since 1990 merger activity in this sector has increased (Munroe, 2005). By 2001 the top ten scientific, technical and medical publishers accounted for 63.4% of the industry; Elsevier Science’s share is 22.9%, Kluwer’s is 11.1% and Thomson’s is 10.7% (Foer, 2005). Adding legal periodicals to the mix places Reed Elsevier first with $8 billion in 2002 total earnings; Thomson second with $7.7 billion; Wolters Kluwer third with $4.02 billion; Springer fourth with its 2004 earnings 880 million euros); Wiley fifth with $854 million; Blackwell sixth with $273 million, and Taylor and Francis seventh with $236.4 million (Case, 2005). As Maskus and Reichman suggest, "the natural competitive disadvantages of follower countries may become reinforced by a proliferation of legal monopolies and related entry barriers that result from global minimum intellectual property standards. Such external restraints on competition could consign the poorest countries to a quasi-permanent status at the bottom of the technology and growth ladder" (2004:2xx). Third, the increasing commercialization of agriculture and medicine means that developing country farmers and the diseases of the poor will be ignored by firms for sound economic reasons (Dutfield, 2003c: 495). As a number of commentators point out, the current system skews research towards rich and middle-income countries' markets and sectors (Barton, 2003; Lettington, 2003; Rai and Eisenberg, 2003). In the public health sector this means the neglect of tropical diseases in favor of cancer and so-called lifestyle drugs (i.e., for obesity, balding, and erectile dysfunction). For example, only 13 of 1233 new drugs marketed between 1975 and 1997 were approved for tropical diseases. “As a result, the rhetoric of strong intellectual property rights leading to innovation that meets social needs rings particularly hollow in this setting” (Hammer, 2002: 888). In the agriculture sector this means concentrating on developing crops unsuitable for subsistence and smallholder farming and not pursuing research beneficial for less lucrative microclimates (Lettington, 2003). There are few private sector incentives to support basic research “that offers only a long-run and uncertain benefit for industry, but is crucial for society” (Barton, 2003: 17). Historically, seed companies preferred to develop hybrids because farmers must purchase new hybrid seed every planting season (UNCTAD-ICTSD, 10 2003: 107). For plant varieties lacking this built-in biological protection, plant breeders can appeal to Plant Breeders’ Rights (PBRs). PBRs have led to increased private investment in agricultural research and have played an important role, particularly in the development of ornamental plant varieties. PBRs "generally do not encourage breeding related to minor crops in small markets" (UNCTAD-ICTSD, 2003: 106). As a result, the private sector under-invests in crops and technologies suitable for smallholder farmers and these public goods are underprovided. Fourth, access to educational materials, drugs and seeds concerns the balance between private rights and public obligations, private ownership and the public domain, and commercial versus humanitarian objectives. Books, drugs and seeds are at the center of a complex political dynamic between stakeholders. Like a three-dimensional chessboard, the institutional and regulatory landscape of these politics is multilayered and multifaceted. For example, just considering the intersection between public health, trade and intellectual property, governance is complicated by the diverse institutions involved, including the World Health Organization (WHO), the International Monetary Fund (IMF), the World Bank, the World Trade Organization (WTO), and the World Intellectual Property Organization (WIPO). Global governance means devising, implementing, and enforcing policies in a way that accommodates a broad range of stakeholders and publics. Challenges to providing effective global governance that balances public interests with private rights include trade pressures, economic coercion, multi-layered governance (i.e., local, national, bilateral, regional, international), the complexity of intellectual property policy jurisdiction across multilateral organizations, the simultaneous development of hard and soft law in diverse venues with conflicting mandates and values, and unequal access to resources and institutions. Among the central features of the quest for global governance in intellectual property are the blurring lines between public and private, the increasing role of the private sector in public policymaking, growing global inequality, constricted autonomy for the weak, and the inappropriateness of “one-size-fits-all” policies for diverse contexts. HOW INTELLECTUAL PROPERTY PROTECTION CAN BE AN OBSTACLE TO ACCESS Intellectual property protection can be an obstacle to access in a number of ways. In recent years intellectual property protection has been dramatically expanded, notably through the WTO TRIPS but also in domestic, bilateral and regional agreements. The baseline for property rights has moved quite far in the direction of private reward over public access. Rights which used to be considered to be privileges or exceptions have superseded obligations of rights holders to the public. A legitimate international intellectual property rights regime must recognize the variegated constellation of interests and abilities within and between countries. A one-size-fits-all approach makes no sense in light of the historical record of economic development. If nations are to craft policies appropriate to their levels of economic development and comparative advantages in innovation and imitation, they must reclaim their autonomy. Every country that has achieved significant levels of economic development began by treating intellectual 11 property policy as tool of public policy. At different historical moments particular state and market actors have defined social efficiency as the public-regarding policy of dissemination and competition. At others, different constellations of state and market actors have defined it as protection and exclusion to encourage innovation (Sell and May, 2001: 472). To insist that all countries adopt high protectionist standards of protection denies them the opportunity to pursue the public policy strategies that every “developed” country enjoyed. Lax intellectual property protection, compulsory licensing, working requirements, keeping certain sectors off-limits in terms of property rights and discriminating against foreign rights holders were all key features of the developed countries’ public policy strategies. Intellectual property rights should be the servant, not the master, of broader public policy goals. The combination of economic concentration and expanded property rights has resulted in higher costs, especially in academic journals and pharmaceuticals. For instance between 1984 and 2001, when the consumer price index of price increases across the economy was 70%, the cost of library subscriptions to periodicals in law rose 205%, in medicine, 479%, and in physics and chemistry 615% (Foer, 2005). Publishers have been earning super profits as a result of their monopolies in academic publishing. Companies have gained increased market share through vertical integration. Cargill is a good example of this dynamic in the agricultural sector: Cargill . . . runs a huge financial services unit, a seed and fertilizer business, is one of the top three beef producers in the U.S. and runs a worldwide transportation business. . . . The market power of companies such as Cargill leave producers as price-takers, forced to accept whatever price Cargill and companies like it are willing to pay. Farmers, the weakest link in the chain, are left accepting prices below their cost of production year after year and cheap produce is dumped on world markets, while corporate profits rise. (IATP, 2005: 8). Patent thickets have been another important problem. Patent thickets have proliferated, in which overlapping patent rights require those seeking to commercialize new technology to obtain licenses from multiple patent holders (Carrier, 2003: 1090-1; see also Rai and Eisenberg, 2003). For instance, when genomics researchers Peter Beyer and Ingo Potrykus sought to make their vitamin A-enriched golden rice available to the International Rice Research Institute (IRRI) they faced significant delays because the technology underlying golden rice was divided among seventy patents held by thirty-two organizations (Phillips, 2004: 181). The transaction costs delayed access for over a year and a half. Additionally, patent thickets can create entry barriers for breeders of new varieties in crop development. Breeders must build upon existing techniques and varieties to develop new transgenic plant varieties. The breeder of a new variety may face a bundle of property rights such as “plant variety rights, patents on plants, as well as several patents relating to transformation technology, the selectable marker employed, the gene coding for the protein, the promoter, and various regulatory elements and modifications needed to express genes adequately in plant cells” (Pistorius and van Wijk, 1999: 148). If a rights holder denies just one element of this package of technologies commercialization of the new variety could be blocked (Pistorius and van Wijk, 1999: 148). Lettington worries that private intellectual property rights could block public sector access to “innovations and 12 germplasm that may be adaptable to smallholder needs and conditions while also limiting public research options due to concerns over unhindered distribution of the products of its research” (Lettington, 2003: 8). Firms seeking to lock in their market positions have also used contract law to extend exclusivity beyond the life of the patent. Perhaps the most notorious such contracts have been Monsanto’s technology use agreements. Monsanto held the patent on glyphosate, a potent weed killer marketed as “Roundup”. Monsanto also had isolated a glyphosate-tolerant gene and developed a variety of “Roundup Ready” (or glyphosatetolerant) crops. In 2000, Monsanto’s patent on glyphosate expired. Now that other firms were free to manufacture and sell glyphosate, Monsanto sought to retain its marketing advantages and protect its investment in glyphosate-tolerant plants. Monsanto developed a technology agreement, a contract between the grower and Monsanto, that stipulated that as a condition of purchasing Monsanto Roundup Ready seed the farmer was obligated to use Monsanto’s Roundup herbicide, to implement an “Insect Resistance Management” program using only Monsanto insecticides, and permit Monsanto to inspect the farmer’s fields for potential seed pirating (Nachtigal, 2001: 58-61). Under these agreements farmers are forbidden to save seeds. The grower may use the seed to plant only one crop “to be sold for consumption by a Monsanto authorized commercial purchaser” (Nachtigal, 2001: 76). In effect, this prevents the farmer from choosing cheaper new forms of glyphosate that appeared on the market after Monsanto’s patent expired. New ways of blocking access to material have emerged in the copyright context. The US Congress passed the Digital Millennium Copyright Act (DMCA) in 1998 that gives legal recognition and protection of digital rights management (DRM). The software and entertainment industry, music and film associations lobbied very hard for protection of works disseminated over the Internet (Yu, 2004: 910). They prevailed over the vociferous opposition of public interest advocates such as librarians, law professors, and electronic civil liberties activists (Vaidhyanathan, 2001: 175). The DMCA offers broad protection for digital works. As Reichman and Uhlir pointed out: In effect, the DMCA allows copyright owners to surround their collections of data with technological fences and electronic identity marks buttressed by encryption and other digital controls that force would-be users to enter the system through an electronic gateway. To pass through the gateway, users must accede to nonnegotiable electronic contracts, which impose the copyright owner’s terms and conditions without regard to the traditional defenses and statutory immunities of copyright law (2003: 378). Further, the DMCA prohibits circumvention of any technological protection against copying and prevents the production of any device or provision of any service designed to defeat protection mechanisms. As Vaidhyanathan argued, for the first time the law “puts the power to regulate copying in the hands of engineers and the companies that employ them. It takes the decision-making power away from Congress, courts, librarians, writers, artists, and researchers” (2001: 174). The use of DRM presents a major challenge to previous practices regarding fair use or, in European terms, fair dealing. Fair use refers to the uncompensated use of copyrighted material for private, educational or research use. It 13 seems likely that the deployment of DRM technologies will consolidate or even worsen the uneven distribution of information and knowledge across the digital divide. The report by the Commission on Intellectual Property Rights (that the British Department for International Development commissioned) concluded that for developing countries, “where Internet connectivity is limited and subscriptions to on-line resources unaffordable, [DRM technologies] may exclude access to these materials altogether and impose a heavy burden that will delay the participation of those countries in the global knowledge-based society” (Commission on Intellectual Property Rights, 2002: 106). The extra controls that subscription online services and copy-protected products allow content owners serve to diminish the hard won compromises for users embedded in doctrines of fair use and fair dealing (May and Sell, 2006: 184).3 It is not just law that can create access barriers. Access blocking technologies are proliferating rapidly in the current environment. In addition to DRM technologies that are protected by law, in the agricultural sector access barriers can exist within the seed itself. The classic blocking technology from the agricultural sector is of course hybrid seed. Hybrid seeds do not breed true to type. A farmer using hybrid seed one year will have to purchase new hybrid seed for the next. The advent of hybrid technologies in the 1920s led to the first major push for property rights in seeds (Fowler, 1994: 52). 1998 ushered in a revolutionary change when Delta and Pine Land, a subsidiary of Monsanto, along with the US Department of Agriculture (USDA) obtained a patent on a copy-protection technique for plants – not unlike DRM in the digital environment. Genetic Use Restriction Technologies (GURTs), a.k.a. “Terminator” technologies, block the plant from replicating itself by rendering harvested seeds sterile. It is a strategic business technology designed “to prevent farmers from replanting saved seed and thereby undercut seed company monopolies” (Dutfield, 2003c: 491). Like DRMs it expands the rights holders’ control of the technology beyond the scope of the law (fair use limits in the former case, patent terms in the latter). Syngenta, DuPont and BASF have been granted or have applied for patents for GURTs in 2003, indicating industry support for pursuing this route to protect seeds from unauthorized replication (van Wijk, 2004: 122). Both the USDA and Delta and Pine Land seek to expand the use of GURTs to increase the proprietary value of US-owned seed companies and to open developing country markets. According to Harry Collins of Delta and Pine Land, co-owner of the patent with the USDA, the patent “’has the prospect of opening significant worldwide seed markets to the sale of transgenic technology for crops in which seed currently is saved and used in subsequent plantings’” (quoted in Dutfield, 2003c: 493). However, if yields from GURT-protected seeds are disappointing, and require greater dependence on expensive agrichemical inputs “then farming communities could suffer both financially and nutritionally” (Dutfield, 2003c: 494). STRUCTURAL, DISCURSIVE AND INSTITUTIONAL DIMENSIONS OF THE POLITICS OF For instance, End User License Agreements (EULAs) set ‘’copyright-plus’ use conditions on publications. EULAs on private software often forbid reverse engineering and other actions that copyright law permits” (Busaniche, 2005), 3 14 ACCESS The development of intellectual property policy is shaped by developments in three dimensions: structural, discursive, and institutional. The structural dimension is characterized by glaring economic and political power asymmetries between developed and developing countries and between the haves and the have-nots in all countries. The discursive dimension involves agenda setting, framing and linking issues and promulgating ideas to mobilize others for change. The institutional dimension highlights the wide and diverse array of organizations charged with addressing intellectual property issues. The structural dimension has fostered the ascendance and consolidation of the global life sciences industries in health and agriculture, and the publishing industry. Battles between commercial and social agendas in intellectual property are hardly waged on a level playing field. Given acute power asymmetries, what are the possibilities available to the weak through discursive and institutional strategies? One of the more striking aspects of the current global order is the fact that governance exists at multiple levels and in multiple forums. This is a double-edged sword insofar as it makes governance more complicated, but it also offers flexibility and new opportunities to shape governance. The “weak” must become adept at playing the multi-level, multi-forum governance game. As Laurence Helfer has pointed out, governments, non-governmental organizations (NGOs), and commercial actors are engaging in “regime shifting” that reveals “an acute awareness by government officials, international secretariats, and non-state actors of the fluidity of lawmaking processes, and reveals such actors’ keen ability to assess the comparative institutional advantages offered by different negotiating fora for achieving particular goals” (2004: 71). Furthermore, stakeholders are fomenting and exploiting “strategic inconsistencies” between institutions, norms and rules to press for different approaches to problems (Raustiala and Victor, 2003: 25). Deftly advancing different types of arguments and mobilizing different players in a variety of international institutions, these actors have begun to alter rules and procedures that affect health, agriculture and access to educational materials. What are the relationships among various international organizations? What perspectives do they promote? Who is defining and shaping these perspectives? When these perspectives conflict, what perspective prevails and why? Are international organizations roughly equal or do they exist in strictly hierarchical relation to each other? How malleable are these relationships? Can discursive and institutional strategies alter these relationships? Global intellectual property rules in the WTO emerged from a vigorous corporate campaign to link intellectual property and trade. This campaign employed economic expertise, effective discursive strategies, and forum shifting to persuade the United States government to redefine its interests in intellectual property protection (Sell, 2003). The result was a dramatic global expansion of protections for rights holders and penalties for violators of intellectual property rights. In the wake of TRIPS, a vigorous civil society campaign, working in concert with a number of developing countries, has mobilized to protest this expansion of rights, particularly in the face of the HIV/AIDS pandemic. It 15 appears that the momentum that the public health activists have generated is contagious. In the past several years the movement has broadened to include campaigns for access to seeds4, educational materials, rights to food security, and rights for indigenous peoples, and the recognition of the contributions of traditional knowledge. Arguing that intellectual property should be construed as a human rights or development issue, rather than a trade issue, these civil society campaigns have scored some victories in challenging the corporate perspective. These campaigns seek to limit the expansion of intellectual property rights and reduce such rights for essential medicines, seeds and educational materials in an effort to contain costs and increase access. TRIPS opponents, consisting of consumer groups, NGOs, and a number of developing country governments, have used “counter-experts” to challenge the trade-based conception of intellectual property rights in favor of a humanitarian perspective. The corporate TRIPS architects have been forced to respond to this challenge and engage the debates of the civil society and developing country groups. The contest among these competing knowledge networks is raging in the WTO over diverse interpretations of TRIPS. Moreover, it is animating deliberations in the WHO, WIPO, the FAO, CBD, and the UNHCHR. Governance in this area is complicated by the fact that different groups are scoring “victories” in some venues, while opposing groups are scoring “victories” in other venues. For instance, while the access to medicines agenda has moved forward in both the WHO and the WTO, industry and those favoring TRIPs-Plus standards have tried to move forward with the Substantive Patent Law Treaty (SPLT) deliberations in WIPO which threaten to reverse any gains achieved in other forums. The United States, at the behest of non-generic pharmaceutical firms, agrichemical companies and copyright-based industries is pursuing an aggressive course of bilateral and regional intellectual property and investment agreements that threaten to undermine any broader gains for developing countries seeking access to drugs, seeds and educational materials. Examining structural, discursive and institutional dimensions of the quest for humanitarian global intellectual property governance helps to highlight both the obstacles to, and the opportunities for, advancing humanitarian or social concerns over competing commercial values when necessary. The international organizations dealing with intellectual property issues are embedded in a broad structural context of unevenly distributed political and economic power. “[P]olicy content has been closely aligned with global shifts in power and influence among key policy actors” (Buse, 2002: 256). The structure of the organizations varies in reflecting these inequalities. For example, an organization like the IMF mirrors unequal power relationships with weighted voting according to monetary contribution, whereas the United Nations General Assembly’s onenation one-vote system formally obscures these inequalities. Furthermore, one of the major developments in the health sector has been “the rapid growth of public-private partnerships in recent years [that] has given the private sector unprecedented entrée into policy-making circles in national governments and key organizations such as WHO, UNICEF, and the World Bank” (Buse, 2002: 262). Access to and participation in various As I demonstrate later in the paper the “seed wars” predated TRIPS, but in the wake of TRIPs the earlier Farmers’ Rights movement has broadened to include Traditional Knowledge. 4 16 organizations is uneven, and the costs of participating in venues such as the WTO can be prohibitive for those without substantial resources (Shaffer, 2004a). Discursive dimensions are also important. Information plays an important role in the policy process. However, information is not knowledge (Comor, 2001: 393). Given bounded rationality, actors employ filters to identify useful and interesting information (Jones, 2001: 26, Tversky and Kahneman, 1981: 435). People transform information into knowledge by employing different normative frames. Frames are “specific metaphors, symbolic representations, and cognitive cues used to render or cast behavior and events in an evaluative mode and to suggest alternative modes of action” (Zald, 1996: 262). Since agenda-setting and advocacy involve both the provision of information and normative frames, they crucially influence policy debates and outcomes (Sell and Prakash, 2004: 145). For instance, casting intellectual property as a trade issue has very different implications than framing it as a food security, public health or human rights issue. According to John Braithwaite and Peter Drahos, “webs of dialogue” can be an important source of change in international politics. “Webs of dialogue” or “webs of persuasion” describe efforts in which actors seek to alter others’ interests. As they suggest, “[i]ssue definition is the first form of persuasion delivered by dialogic webs that is a prerequisite for a global regime” (Braithwaite and Drahos, 2000: 553). Indeed, in their survey of global business regulation, they conclude that webs of dialogue catalyze change much more frequently than do webs of coercion. Global governance in intellectual property protection, as exemplified by TRIPS in the WTO, has come about by the mechanism of coercion (Abbott, 2002a: 469). However, in the aftermath of TRIPS, and the contest between those who frame intellectual property as a trade issue and those who frame it as a human rights and access issue, webs of dialogue are becoming increasingly important. As Braithwaite and Drahos point out, “[d]ialogic webs offer individuals the possibility of micro action to secure macro change” (Braithwaite and Drahos, 2000: 7). Competing knowledge networks participate in dialogic webs in their efforts to define issues, persuade others to redefine their interests, and inject normative commitments that shape prescriptions and seek to persuade others that “compliance is morally right” (Braithwaite and Drahos, 2000: 553). Authors working in the rational choice tradition focus on processes given preferences. As Patrick Jackson has pointed out, rationalist accounts are silent on the issue of legitimacy; “[w]hat vanishes from sight . . . are any notions of persuasion, learning, reflective reconsideration, or any of the other activities that go on when a leader tries to render a policy acceptable to an audience” (Jackson, 2002: 735, 744). Authors concerned with questions of legitimacy and negotiation processes focus on the content of actors’ beliefs and principled argument (Albin, 2001: 1-3; Coleman and Gabler, 2002: 481; Crawford, 2002: 11-14; Keck and Sikkink, 1998: 1-8;; Muller, 2001: 160). Principled argument used in dialogic webs has the potential to alter actors’ interests and outcomes. While coercion is a viable weapon of the strong, principled argument can be a potent asset for the weak to bring about desired change—especially when coupled with the strategic use of institutions. As two critics of the access campaigns stated, “if ideas matter, then the proponents of the New International IP Agenda should be considered formidable. The NGOs pushing this agenda are wellfunded, well-organized and smart. They are also persistent, as they have proposed to curtail 17 intellectual property rights in one international forum after another, even where IP was not the main issue” (Schultz and Walker, 2005: 82). The institutional dimension concerns the broad range of different multilateral institutions that are promulgating laws, declarations, resolutions, hard and soft law related to global public health, agriculture and educational resources. These institutions reflect competing conceptions of which values should be promoted. Since WTO law is binding and enforceable, it is imperative to examine its role and its relationship to other institutions to assess the prospects for and obstacles to global governance in intellectual property. International law scholars also address the institutional dimension in a lively debate about the boundaries of the WTO and the extent to which declarations, laws, and regulations promulgated in other venues are relevant to WTO deliberations. It also addresses the question of what role the WTO should play in resolving politically-charged value conflicts. This issue is central to conflicts over intellectual property because a variety of different international organizations have weighed in on the matter, and these organizations reflect competing and conflicting conceptions of intellectual property. Competing values are at stake both outside of and within the WTO. For example, WTO judicial panels hearing cases involving pharmaceutical patent protection face a major dilemma. [They] cannot simply recognize a public good in interpreting the TRIPS Agreement. [They] must rather take account of concerns over competing public goods as reflected in the agreement’s provisions The ultimate issue in choosing among the production of public goods becomes institutional because different institutions offer different opportunities for actors to participate, affecting which perspectives on the appropriate balancing are advanced (Shaffer, 2004a: 464). This raises an urgent problem of which perspectives should have pride of place in interpretations of states’ rights and obligations. STRUCTURAL DIMENSIONS The contemporary global intellectual property regime is embedded in a broad structural context characterized by asymmetrical power relationships (Sell, 2003). Over the past thirty years, the globalization of finance and the ideological shift represented in the early 1908s by the Reagan and Thatcher revolutions' embrace of unfettered faith in the marketplace has increased transnational corporations’ power vis-à-vis the state. States, seeking to be globally competitive, have liberalized their markets, engaged in deregulation and privatization, and have implemented new regulatory structures designed to promote efficiency and enforce market-friendly behavior. According to Cerny, “the institutions of the state itself are increasingly marketized or ‘commodified’, and the state becomes the spearhead of structural transformation to international market norms both at home and abroad” (Cerny, 2000: 304). States have increasingly commodified activities by putting once-public activities into the market. Prominent examples include the privatization of prisons, hospitals, military support services, and even “mission-critical” functions such as providing protection for head of the Coalition Provisional Authority, L. Paul Bremer III, in 18 the U.S. occupation of Iraq. The expansion of intellectual property rights, and the privatization of federally funded research under the Bayh-Dole Act5, must be seen as an instance of this larger trend. These broad economic changes have had profound effects on developing countries. Earlier models of economic development such as import-substituting industrialization once-popular in Latin America and India became discredited through economic stagnation and the debt crises of the 1970s and 1980s. The success of the East Asian "Tigers" vindicated export-led development and integration into global markets. Many developing countries subsequently reversed decades-old policies of economic nationalism in favor of market liberalization and privatization, and consequently slashed public budgets. Developing country governments began to compete to attract foreign investment and eased former restrictions of foreign investors' activities. The new push toward export-led growth meant that developing countries needed access to industrialized country markets. Their trade dependence upon such countries, particularly the United States, gave the United States considerable economic leverage over developing countries seeking access to its large market. Using Super 301 of the U.S. Trade Act, the USTR, at the behest of hightechnology firms, threatened trade sanctions against developing countries unless they adopted and enforced highly protectionist intellectual property policies. Such economic coercion was an important factor behind developing countries' ultimate acceptance of TRIPS. This liberalizing agenda favors finance capital and other mobile factors of production (Baker, 2000: 364). Transnational firms in knowledge-intensive sectors such as pharmaceuticals, chemicals, software, and entertainment "have the resources, motivations 5 The 1980 Bayh-Dole Act allowed “grantees to seek patent rights in government-sponsored research results” (Rai and Eisenberg, 2003: 290). The idea behind this was that many inventions with commercial potential lay fallow in university laboratories, and that patenting opportunities would give universities incentives to “scour” research labs for significant and marketable inventions (Lieberwitz, 2004). This was part of a larger governmental quest in the late 1970s and 1980s to pursue economic competitiveness vis-à-vis Japan in particular. The Bayh-Dole Act has prompted a flurry of university patenting activity, at least a ten-fold increase since 1979 (Rai and Eisenberg, 2003: 292). The Bayh-Dole Act has had beneficial effects in generating revenue for cash-strapped public universities. For instance, the $300 million patent infringement award that the University of Minnesota won for the development of the drug Ziagen has provided muchneeded funding for research and graduate student support. University patent portfolios also help to attract private sector funding, especially in biotechnology. However, the Bayh-Dole Act has also created new divisions within universities. As Rai and Eisenberg point out, the legislation makes no distinction between upstream and downstream research, and as a result more and more research tools have become patent-protected (2003: 290-1). An unintended consequence of the Bayh-Dole Act has been the dramatic reduction of open access to research tools. Technology transfer offices are charged with patenting and licensing technology to generate revenue for the institution. Research scientists are more interested in having access to “open science” (Rai and Eisenberg, 2003: 305). In copyright, the parallel is between university presses seeking to generate revenue, and educators seeking to maximize access to copyrighted works. Bayh-Dole has also increased university collaboration with private sector biotechnology firms, and has raised many questions about academic freedom, research priorities, and incentives. Some critics have gone so far as to assert that universities have lost their sense of public mission (Lieberwitz, 2004). 19 and capabilities to roam the world searching for the kind of opportunities that promise lucrative rewards" (Germain, 2000: 81). These privileged sectors participate in “globalized” markets in so far as “there are a small number of participants who know one another and operate across countries with a common conception of control” (Fligstein, 1996: 663). TRIPS reflected the wishes of these privileged sectors and globalized their preferred conception of control by establishing a high standard of intellectual property protection. Beyond extending property rights, competitiveness concerns moved the United States to relax its antitrust policies. The Reagan Administration codified this approach in the Antitrust Division's Merger Control Guidelines of 1982. Reflecting the influence of the Chicago School of Economics the new guidelines abandoned a populist focus on market structure in favor of the Chicago school's focus on price theory. In this view anticompetitive business practices are those that reduce output and increase prices; business practices that expand output are pro-competitive (Sell, 1998: 158). In contrast to earlier approaches, according to the Chicago school, "high levels of market concentration and the exercise of market power may be indicative of efficiencies" (Eisner, 1991: 105). The 1982 guidelines presented an expanded definition of relevant markets; this had permissive effects. The guidelines allowed for the introduction of non-structural factors, such as foreign competition or the possession of new technology that was important to long-term competitiveness (Eisner, 1991: 198). The Justice Department argued that "anti-trust law should not be applied in a way that hinders the renewed emphasis on competitiveness" (Hoff, 1986: 19). The consequence of this new thinking was to remove most intellectual property licensing from antitrust scrutiny. Under the Reagan administration, "the executive agencies viewed the economic incentives provided by intellectual property rights as legitimate means of extracting the full economic benefit from innovation. Intellectual property rights acted as a 'magic trump card' allowing many previously suspect arrangements to proceed without challenge from the FTC or DOJ" (Hayslett, 1996: 382). Furthermore, intellectual property rights have been dramatically expanded in recent years to cover things such as computer programs, compilations of data, genes, entire plant species, software algorithms, pharmaceutical products and processes, and “practices in local agriculture, medicine and education which were outside of market relations” (Arup, 1998: 367-81; see also Correa, 2002: 550). This combination of relaxed antitrust policies and expanded intellectual property rights has promoted economic concentration in high technology sectors, and particularly in the life sciences industries As Maskus and Reichman point out, “weak enforcement of antitrust laws then further reinforces the barriers to entry erected upon this thicket of rights, while the need to stimulate and coordinate investment in complex innovation projects justifies patent pools, concentrations of research efforts, and predatory practices formerly thought to constitute misuses of the patent monopoly" (Maskus and Reichman, 2004).The consequences include enhanced political power of these industries, a reduction in the number of suppliers of certain kinds of technology, a reduction in competition, and higher costs of technology. As Assad Omer suggests: 20 Developing countries are confronted with the following dilemma: on the one side, in order to attract more investment and technology they have to press to open up their markets, and on the other side, the reduction of regulatory barriers gives rise to the emergence of anti-competitive behaviour of firms (Omer, 2001: 312). Even more ominously, Drahos and Braithwaite argue that: The globalization of intellectual property rights will rob much knowledge of its public good qualities. When knowledge becomes a private good to be traded in markets the demands of many, paradoxically, go unmet. Patent-based R&D is not responsive to demand, but to ability to pay. . . . Much of what happens in the agriculture and health sectors of developed and developing countries will end up depending on the bidding or charity of biogopolists as they make strategic commercial decisions on how to use their intellectual property rights” (2002: 167168). The structural power of global firms is reflected in the membership of key policy making committees in US trade institutions. These committees assist US trade negotiators in designing policies for multilateral, regional and bilateral trade. The United States Trade Representative’s (USTR) agricultural Trade Advisory Committee represents these corporations: Altria, Archer Daniels Midland, Blue Diamond Growers, Burger King, Campbell Soup, Cargill, ConAgra, General Mills, Hershey Foods, HJ Heinz, Land O’ Lakes, Louis Dreyfus, McDonalds, Monsanto, Sunkist Growers, Sun Maid Growers, WalMart, and Yum! Restaurants.6 The USTR’s Industry Trade Advisory Committee on Intellectual Property Rights includes representatives for Pfizer, Eli Lilly and Company, PhRMA, Merck & Company, Inc., Biotechnology Industry Organization, Time Warner, Inc., International Anti-Counterfeiting Coalition, Recording Industry Association of America, Intellectual Property Owners Association, John Wiley and Sons, Inc., and Association of American Publishers. None of these firms or organizations is pressing for more balance between private rights and the public domain. The reach of these advisory committees can be quite broad. For instance, ActionAid reported that the most influential non-governmental organization in Brazilian agricultural trade policy is the Institute for the Study of Trade and International Negotiations (ICONE). ICONE is supported by the Brazilian Agribusiness Association that is funded by Cargill, Archer Daniels Midland, Bayer, Monsanto and Pioneer Hi-Bred. “Issues such as protecting food security and poor farmers’ livelihoods are not on its agenda” (ActionAid, 2006: 30). US-based firms work with their subsidiaries abroad to develop support for their positions. Structural features at the macro level shape the micro level, as manifest in the under-provision of drugs for tropical diseases, public health budgets reduced under IMF conditions, and the constrained ability of governments to meet public health needs. “Increasingly [IP] rights have invaded the research commons itself and made it both costly and difficult to obtain cutting-edge technologies needed for public health, agricultural production, environmental protection and the provision of other public goods” (Maskus 6 Yum! Brands is a group of US fast food chains including Kentucky Fried Chicken, Pizza Hut and Taco Bell. In January 2005 Yum! formed the Food Trade Alliance to lobby the American government to liberalize agriculture in the WTO. The Alliance seeks the cheapest prices for farmers’ goods and the broadest market access possible (ActionAid, 2006: 30). 21 and Reichman, 2004). Market incentives are insufficient for stimulating agricultural products suited for smallholder farmers in developing countries. DRMS and monopolies in academic publishing reduce access to educational materials. Weak regulatory frameworks in low and middle income countries confronting global life sciences markets and consolidated publishing markets leave their populations particularly vulnerable (Buse, 2002: 260). Focusing on the formal features of intellectual property law, texts and institutions, one sees plenty of room for state discretion and flexibility in adapting the global minimum standards to local concerns. However, this formal universe is embedded in a system of asymmetrical power relationships and global capitalism that constrain weaker states' abilities to take advantage of the flexibilities crafted into the law. In both the medicines issue and agriculture, developing countries have not taken full advantage of TRIPS flexibilities. This is largely because they are eager to attract foreign investment and are concerned about alienating potential foreign investors. They also are eager to have access to technologies that may help them to further develop, provide reliable nutrition, and address a myriad of pressing problems. Most of these countries lack significant bargaining leverage and the capacity to resist the high-pressure tactics of the USTR and the industries that it represents. DISCURSIVE DIMENSIONS OF AGRICULTURE The battle over agriculture revolves around a number of related issues. In examining the regulatory environment the central question is how much discretion do states have in limiting intellectual property rights to support smallholder agriculture and environmentally sustainable agricultural practices? Critics of the increasing commodification of what was once treated as the public domain have raised at least seven issues of concern: 1) threats to traditional agriculture and food security ; 2) abuses of monopoly power; 3) increased dependence on costly commercial agriculture; 4) threats to biodiversity; 5) "biopiracy"; 6) questions of benefit sharing; and 7) the status of traditional knowledge. Agricultural issues attracted substantial attention in the early 1980s during the socalled “seed wars” in which activists protested patents on living organisms (such as plant varieties) first in the US and Canada, and then in the Food and Agriculture Organization. At that time activists expressed concern about genetic erosion and the dangers that property rights in plant varieties posed to traditional farmers’ rights and smallholder agriculture. Farmers’ rights to save and exchange seeds came under increasing attack with expanded property rights over seeds and germplasm. In what Pistorius and van Wijk have called the “third agro-food order” crop development conglomerates overtook the state in dominating agricultural research, crop development and dissemination of seeds (1998: 105). Rather than emphasizing national food needs, these conglomerates emphasize saving the world from food shortages (Pistorius and van Wijk, 1998: 125). Their conservation strategies include securing long-term access to biological material and promoting strengthened property rights to safeguard their R&D investments (Pistorius and van Wijk, 22 126). Large scale agribusiness promotes mono-cropping and uniform seeds that deplete the soil over time and heighten dependence on the conglomerates to provide the seed and inputs. Those opposed to IP protection and private rights over plant varieties fear their loss of control over the use of their plant genetic resources. They fear that the use of their traditional varieties may be prohibited if they carry genes covered by a patent (Pistorius and van Wijk, 1998: 183). According to C. Montecinos, of the Centro de Educacion y Technologia in Santiago Chile, ‘The only way to fight those [northern industrial] monopolies is by fighting megamarkets. This will happen when we reclaim diversified production systems based on local resources and knowledge, when agriculture ceases to be an inputconsuming machine, when farmers reclaim the right to use and develop their own technology, when we stop eating the same thing in Manila, Pittsburg and Concepcion, and our health no longer depends on Monsanto and Ciba Geigy. This will also be the only way in which indigenous peoples and farmers will become the primary beneficiaries of what their societies have created and continue to create’ (quoted in Pistorius and van Wijk, 1998: 184). While not all farm-oriented NGOs and indigenous peoples’ groups support this total rejection of the monopolies, they do promote alternative agricultural production strategies “directed at the autonomous improvement of subsistence conditions in the poorest rural areas” (Pistorius and van Wijk, 1998: 184). They argue for support for organic farming, low-input oriented farming, the use of landraces and on-farm saved seed, and biological insecticides as viable alternatives (Pistorius and van Wijk, 1998: 194). Biopiracy is a second major area of contention. In the lead-up to TRIPS American and European negotiators invoked the language of “piracy” and “theft” to label what were in fact perfectly legal practices by foreigners. Developing countries routinely were accused of stealing American and European intellectual property. The rhetoric of biopiracy has turned the “piracy” rhetoric back on industrialized countries (Shiva, 1997). It accuses them of stealing the genetic diversity and genetic resources of the geopolitical South, patenting these resources and/or traditional knowledge, and then selling the products back to them at exorbitant prices. Activists also underscore the danger that the people who have used these genetic resources and/or techniques for ages may be denied using them with the introduction of property rights over these held by foreigners. Another area of concern is the terms of access to such resources and whether foreigners using these resources should be required to disclose the origin of the resources in their patent filings. Many developing countries are pressing for disclosure requirements and the establishment of a benefit sharing regime in which indigenous peoples would be compensated for use of the resources and would be entitled to a share of any royalties earned by commercializing the derived products. Finally, a number of developing countries argue that traditional knowledge, including plant genetic resources, must be protected from bio-pirates. While the means for doing so are highly controversial (Kuanpoth, 2006: 13952), some argue that they should assert intellectual property rights over traditional knowledge and folklore. Others promote the establishment of databases to document and catalog traditional knowledge to make it more transparent when it is included in a patent application. This would assist patent examiners in establishing prior art. Still others reject 23 applying property rights and commodifying traditional knowledge at all. Not surprisingly, the agrichemical and biotechnology industry has a different perspective on the issues. The life sciences conglomerates resist any attempt at being forced to include disclosure of origin in patent applications. They argue that it would inject unacceptable levels of uncertainty into the process of commercializing biotechnology. They argue that this uncertainty would have a chilling effect on investment in bio-prospecting (Gorlin, 2006: 4). Industry views these agriculture issues as investment issues. Since the 1980s when it linked intellectual property to trade, it underscored a strong relationship between high levels of intellectual property protection and foreign investment. Given that many developing countries are eager for foreign investment, industry leverages this vulnerability to press its agenda (Correa, 2004: 345-46). It prefers contractual approaches such as the Merck-InBio deal and the Novzymes/Biotec access and benefit sharing agreement in Thailand (ABIA, 2005) in which firms underwrote bio-prospecting programs and agreed to share a percentage of any royalties derived from commercialization of the genes found. Technological, judicial and legislative changes together have produced a radical shift from public to private provision of seeds. As Aoki points out, “the private seed market for grains was almost non-existent at the beginning of the twentieth century, due to free government seed distribution and the widespread practice of farmer seed saving” (Aoki, 2003: 268-9). Farmers traditionally have saved seeds and reused them. They traded seeds with each other, sold seeds to each other, and created and experimented with new hybrids. In these ways they have contributed to the planet's biodiversity. In the past, US laws covering plant varieties incorporated the concept of farmers' rights in which farmers retained their freedom to engage in these important and traditional activities. Legislative changes extending property rights in plant varieties, such as the 1970 adoption of the United States Plant Variety Protection Act, “increased the expectations of seed industry profits and thereby helped to stimulate an upsurge in mergers and acquisitions” (Dutfield, 2003a: 149). Furthermore, in August 1994 the US Congress amended the Plant Variety Protection Act and removed the farmer's exemption so that now “it is expressly illegal for farmers to sell or save seeds from proprietary crop varieties without receiving permission from breeders and paying royalties” (Shulman, 1999: 90). Ironically, according to Aoki, the commodification of germplasm has transformed the US patent office (PTO), “which in the early 19th century began to collect and catalog and make freely available seeds”, into “by the beginning of the twenty-first century, a primary means by which the ages-old practice of farmer seed-saving is under serious and vociferous legal attack” (Aoki, 2003: 331). In Aoki's words, “the intersection of biotechnical knowledge and methods and expanded legal protections for plant breeders transforms seed germplasm into a paradigm commodity” (Aoki, 2003: 261). Life sciences corporations “emerged out of a wave of mergers, acquisitions, joint ventures and strategic partnerships involving companies in a wide range of fields such as chemicals, seeds, processed foods, dietary supplements and pharmaceuticals” (Dutfield, 2003a: 147-8). Advances in biotechnology spurred the consolidation process throughout the 1970s and especially the 1980s. The 1973 development of the recombinant DNA 24 technique, which enabled foreign genes to be inserted into microorganisms, launched the era of commercial biotechnology (Dutfield, 2003a: 138). It is noteworthy that while the path breaking Cohen-Boyer method for combining DNA from different organisms was patented, “the patents were licensed non-exclusively and cheaply to encourage firms to take licenses rather than to challenge the patents” (Rai and Eisenberg, 2003: 300). This technology had been federally funded, and “many observers attribute the rapid progress of the biotechnology industry to the fact that this technology was made widely available rather than licensed exclusively to a single firm” (Rai and Eisenberg, 2003: 300). In 1980 the US Supreme Court ruled in Diamond v. Chakrabaty (447 U.S. 1980) that a man-made oil eating bacterium could be patented. This case led to the expansion of rights to own living organisms and injected greater certainty into the development of commercial biotechnology. The ability to acquire patents on altered life forms helped biotechnology start-up companies to raise venture capital. With the advent of genetic engineering, plant breeders sought even stronger protection of their investments by pursuing patent protection. TRIPS ultimately placed restrictions on patenting of life forms (to the dismay of the American biotechnology industry). Article 27 permits the exclusion of plants and animals from patentability, but 27.3(b) requires that members provide protection for plant varieties or an “effective sui generis” (uniquely tailored) system. However, there really is no consensus on what a sui generis system needs to include, and as Sutherland points out, the negotiating history of Article 27 provides little guidance because it provides no record of the meaning of sui generis (Sutherland, 1998: 295). American intellectual property industry activists have been pushing the Union for the Protection of New Varieties of Plants (UPOV) as the model sui generis system. UPOV, to which fifty (UPOV 2002) mainly industrialized countries subscribe, was last amended in 1991. The 1978 version of UPOV provided two limitations on the monopoly rights of plant breeders. First, other breeders could freely use UPOVprotected varieties for research purposes. Second, farmers could reuse the seed for the following year's harvest under certain conditions. The 1991 revision “narrowed down the exemption for competing breeders and it deleted the so-called farmer's privilege... [It] extends the breeders' monopoly right to the product of the farmer's harvest” (GRAIN, 1999: 2). “Although the UPOV system allows on-farm replanting, its rules restrict farmers' freedom to buy seed from sources other than the original breeders” (UNCTAD-ICTSD, 2003: 107). UPOV91 “does not authorize farmers to sell or exchange seeds with other farmers for propagating purposes” (Helfer, 2002: 17). Additionally, before the adoption of UPOV91, plant breeders were forced to choose to protect their plant varieties with either a plant breeder’s right (PBR) or a patent. UPOV91 “removed the 1978 Act's ban on dual protection and now permits member states to protect the same plant variety with both a breeders' right and a patent” (Helfer, 2002: 15). Grassroots activists are convinced that American industries are seeking this same approach through TRIPS by pushing a particular interpretation of sui generis protection under Article 27.3(b). Any country wishing to join UPOV today must sign the 1991 treaty. It is very generous to the corporate plant breeder. Countries eschewing the UPOV system, can adopt sui generis systems of protection that allow “farmers to acquire PBR-protected seed from any source and/or requiring protected varieties to display qualities that are genuinely superior to existing varieties” (UNCTADICTSD, 2003: 107). 25 A number of developing countries have favored a sui generis approach that protects them from biopiracy and explicitly honors farmers’ rights. Laurence Helfer has analyzed developing countries’ options under 27.3 (b) to assess how much policymaking discretion follows from each option. States that adopt TRIPS and ratify or accede to UPOV91 have the least policy flexibility (Helfer, 2002: 37). While corporate plant breeders would prefer that developing countries adopt UPOV91 as their domestic legislative standard, these countries are by no means required to do so. States wishing to retain maximum flexibility and discretion to serve the needs of smallholder agriculture would be well advised to adopt TRIPS only (Helfer, 2002: 37). As John Barton suggests, "the chances are that for a poor nation, neither a UPOV nor a regular patent approach will actually encourage privatesector research. Hence, such a nation is probably best-off adopting minimum compliance with TRIPS" (2003: 12). TRIPS’ Article 27.3(b) preserves “significant leeway for national governments to work out the precise manner in which they will balance protection of IPRs against other international obligations and national objectives" (Helfer, 2002: 31). TRIPS, unlike UPOV91, preserves the right for subsistence farmers to exchange seed. States are free to incorporate both subsistence farmer exemptions and research exemptions in national plant breeders’ rights legislation (Barton, 2003: 23-24). Countries wishing to adopt the stronger UPOV91 system should consider incorporating waivers for subsistence farmers or smallholder exemptions (Barton, 2003). In countries lacking in significant private sector competition, as is often the case in poor countries, public sector seed provision will be important to promote competition to stimulate both variety and lower prices (Barton, 2003: 14). In developing countries a large number of farmers are smallholders, who do not participate in the market sector in any substantial way. Indeed, in developing countries “80% of the total seed supply is produced on farm” (van Wijk, 2004: 121). Smallholder farmers engage in seed-saving, replanting, and "across-the-fence" exchange. This is especially in countries (many in Africa) where neither the public nor the private sector plays a significant role in producing or distributing seed" (UNCTAD-ICTSD, 2003: 107). The smallholder farming sector plays an important role in contributing to national food needs. "Small holder farmers produce fifty-one percent of Latin America's maize, seventy seven percent of its beans and sixty one percent of its potatoes while in Africa they produce the majority of grains and legumes and almost all root, tuber, and plantain crops" (Lettington, 2003: 13). For example, in Peru between 50-60%, and in Kenya between 7080% of the population depends on smallholder agriculture for their livelihood (Lettington, 2003: 13). In a comparative study of smallholder farming in Peru and Kenya, Lettington did not find evidence that Plant Variety Protection (PVP) legislation harmed smallholder agriculture. However, he argued that “the current system of PVP legislation is failing to create solutions to existing problems” (Lettington, 2003: 32). In particular, it creates incentives that direct energy away from catering to subsistence farmers' needs in favor of 26 large commercial agricultural enterprises. It also facilitates the promotion of the use of commercial seed (as opposed to landraces or “wild” cultivars). “The end result has been a hastening of the deterioration of food security in these areas” (Lettington, 2003: 34). Lettington suggests that governments that seek to limit the cost of seed in economically and climatically marginal areas may “need to place limits on the nature of intellectual property rights” (2003: 34). Lettington recommends that: The activities of smallholder farmers, in particular the saving, use, exchange, and sale of farm-saved seed, should be explicitly stated as not subject to the rights of intellectual property rights holders. In accordance with the purposes and objectives of TRIPS, effort should be made to develop effective incentives for research targeted at smallholder farmers.... Limited exceptions to intellectual property rights should be permitted to promote the adaptation of protected products to the needs of smallholder farmers. These should apply to both research and development and to manufacturing and distribution (2003: 8). DISCURSIVE DIMENSIONS OF HEALTH The battle over access to essential medicines revolves around the rights to issue compulsory licenses and to manufacture and export generic versions of brand name drugs. Global brand name pharmaceutical corporations seek to restrict the ability of generic manufacturers to produce and distribute essential medicines. African countries in the grip of the HIV/AIDS pandemic, Brazil, India, and their NGO advocates seek to clarify interpretations of TRIPS that permit compulsory licensing, parallel importing, generic manufacture and export. The debate over TRIPS and access to medicines has galvanized a broad range of stakeholders. Brand name pharmaceutical companies (a.k.a. global pharma), developed and developing country governments, the USTR, NGOs representing public health and consumer interests, and generic drug manufacturers are all participating in this vigorous debate. Among the competing values embedded in TRIPS are the generation of knowledge, the facilitation of “undistorted” trade, and the protection of public health (Shaffer, 2004: 460). On one side of the TRIPS and access to medicines debate are those who support strong intellectual property protection for pharmaceuticals and argue that, if anything, TRIPS is too weak. These advocates highlight the high costs of developing new drugs, the importance of strong property rights as incentives for innovation, and the need for substantial compensation for providing life saving drugs (Grabowski, 2002: 850-53). This view is most prominently associated with the brand name global pharmaceutical industry, the United States, and the USTR. It has also been influential in the WTO. The industry fears that any expansion of cut-rate drugs will undermine its markets, particularly if they find their way into high income industrialized country markets. Global pharma highlights the potential health dangers of widespread generic production, “piracy,” and the use of drugs without the supervision, dosing instructions, and regulatory controls covering global pharma’s products (Symposium, 2002: 729). 27 Perhaps the most frequently offered argument from supporters of global pharma is that the big problem is not patents but poverty (Calfee, 2003; Bate and Tren, 2003). This view has been promulgated in industry-supported American think tanks such as the American Enterprise Institute and the International Intellectual Property Institute (IIPI). Roger Bate and Richard Tren presented their remarks at an American Enterprise Institute forum on “unelected” NGOs. The United States-based Pharmaceutical Research and Manufacturing Association (“PhRMA”), an industry-lobbying group, is hardly subtle about its efforts to enlist academics to promote its cause. The Washington Post has referred to these as “hall-of-mirrors techniques by which special interests amplify their arguments through seemingly unconnected third parties” (Washington Post, 2003, June 9). For example, for 2005, PhRMA budgeted $1 million for an: [I]ntellectual echo chamber of economists – a standing network of economists and thought leaders to speak against federal price control regulations through articles and testimony.” It has set aside $550,000 “for placement of op-eds and articles by third parties” and at least $2 million for outside research and policy groups “to build intellectual capital and generate a higher volume of messages from credible sources” backing industry positions. Overall, the group will devote $12.3 million to “alliance development,” . . . with . . .economists, doctors, patients, and minority groups (Washington Post, 2003). PhRMA frequently cites a “Harvard study” that “proves” that patents are no obstacle to access to antiretroviral medicines in Africa (Attaran and Gillespie-White, 2001: 1888-91). Amir Attaran was an adjunct lecturer in public policy at Harvard, and his coauthor, Lee Gillespie-White, worked for a PhRMA-supported think tank International Intellectual Property Institute (IIPI). The oft-cited paper originated as a study that PhRMA commissioned with its think tank (IIPI) headed by Bruce Lehman, former United States Commissioner of Patents (Abbott, 2002a: 485, n. 62). The United States trade delegation relied on this then unpublished study in its Talking Points in late September 2001 in the run up to the WTO Doha Ministerial meeting (Abbott, 2002a: 485). Substantively, advocates of PhRMA’s position object to any weakening of intellectual property protection through public health exceptions. They reject compulsory licensing as a policy tool to bring the costs of essential medicines down. They reject parallel importing (Symposium, 2002: 727), whereby states can take advantage of differential pricing policies and import the cheapest version of the brand name patented pharmaceutical product. Overall, they ardently oppose any efforts to weaken the international system of intellectual property protection. Instead, they advocate increased foreign aid, drug donations from firms, and “protection for international price discrimination against the threat of ‘grey market’ arbitrage” (Bloche, 2002: 838). On the other side of the debate is an alliance of developing country governments and NGOs campaigning for access to essential medicines. They argue that patent protection is a barrier to access and that public health exceptions to patent rules are necessary to prevent needless deaths. They advocate compulsory licensing, generic competition, and fixed rates of compensation for pharmaceutical companies. 28 Among the most outspoken advocates of this position are James Love of American consumer activist Ralph Nader’s Consumer Project on Technology (CPTech), and Ellen ‘t Hoen of Médecins Sans Frontières (MSF). They consistently have attacked PhRMA’s positions on these issues. Ellen ‘t Hoen points to strong intellectual property protection as one important barrier to access; she argues that patent protection leads to high prices and limited access (‘t Hoen, 2002: 29). MSF and other NGOs have expressed a number of concerns about TRIPS, including high drug prices, reduced availability of quality generic alternatives, inadequate research and development into tropical diseases, and bilateral pressures on developing countries to adopt patent protection that exceeds the requirements of TRIPS (‘t Hoen, 2002: 29-30). Furthermore, Love has challenged PhRMA’s claims that its companies spend $500-800 million developing each new drug. Love has argued that the majority of important HIV/AIDS drugs were actually developed by the public National Institutes of Health (NIH), and funded by taxpayers’ dollars (CPTech, 2000). Love and others have also criticized the Attaran and Gillespie-White 2001 argument (Symposium, 2002: 732-5; CPTech et al., 2001). Brazil, India, and the African group of countries have been leaders in the intergovernmental efforts to address their public health emergencies. As José Viana, a Brazilian trade delegate, remarked, “[t]he Brazilian government has consistently supported the idea that public health should not be subordinate to abuses of economic power” (Viana, 2002: 311). Activists have praised Brazil’s policies of providing universal access to HIV/AIDS drugs (Rosenberg, 2001: 26). Brazil has used the threat of compulsory licensing7 to negotiate steep drug discounts with global pharma. It also has committed resources to producing generic drugs. Its policies have helped to create a market for high quality generic drugs (Symposium, 2002: 702). Creating a market has encouraged competition that has brought HIV/AIDS drugs prices down from $10,000 to $150 a year per patient.8 As a WHO report concludes, “[c]ompetition is perhaps the most powerful policy instrument to bring down drug prices for off-patent drugs” (Abbott, 2002a: 472 n. 702). Above all, the access to medicines campaign endorses the right of developing countries to compulsory license drugs, to produce and export generic drugs, and to take advantage of parallel importing to seek out the lowest cost medicines. INSTITUTIONAL DIMENSIONS:THE WTO Ever since the Uruguay Round of trade talks ended in 1993, a number of World Trade Organisation (WTO) members have pressed for clarifications of TRIPS. Developing country members have engaged in sharply pitched battles with developed country governments over broken promises and unfinished business. TRIPS requires all WTO members to increase protection and enforcement of intellectual property rights. When negotiating parties agreed to TRIPS it was part of a quid pro quo in which developing countries agreed to high standard intellectual property 7 8 Seizing the patent But see MSF on the continued high costs of second-line therapies, www.msf.org 29 protections in exchange for access to developed countries’ agricultural markets and the liberalization of developed countries’ agricultural sectors. In the wake of the Uruguay Round developed countries have failed to make good on their promises to cut domestic supports for agriculture, while they aggressively have pursued WTO action against developing countries for failure to implement intellectual property policies. They also have pursued a vigorous campaign of bilateral and regional investment and trade agreements that incorporate “TRIPS-Plus”9 standards of intellectual property protection. However, Brazil successfully challenged the United States over its cotton subsidies; the WTO ruled in Brazil’s favor in May 2004 (ICTSD: 7). Brazil is considering placing sanctions on U.S.held intellectual property (cross-retaliation) if the U.S. does not meet its deadline to cut cotton subsidies. At the Doha WTO Ministerial meeting in Qatar in November 2001, member states launched a so-called “development round”. The most pressing intellectual property issues in the Doha Development agenda address public health, biopiracy, indigenous people and traditional knowledge. For example, member states adopted the Doha Declaration on TRIPS and Public Health that stated that nothing in TRIPS should interfere with member states’ ability to provide public health. Today, many developing countries are focusing on the unfinished business of the Doha Round. In addition to agricultural market access, the unfinished business in intellectual property includes: the ability of developing countries to export generic drugs made under compulsory license; the relationship of TRIPS to the Convention on Biological Diversity (CBD); provisions to protect plants and genetic material located in developing countries from biopiracy; benefit sharing arrangements; implementation; and the expansion of geographical indications. All but the last item are central to the Doha development agenda, although the EU is pushing hard to expand geographical indications. The acrimonious ending to the Cancun WTO Ministerial meeting in August 2003 put the negotiating spotlight on agriculture, but intellectual property issues received extra attention in October 2005 in the face of a global threat of an avian flu pandemic. INSTITUTIONAL DIMENSIONS, THE WTO: SOCIAL ISSUES AND LEGAL DEBATES Since the WTO administers TRIPS, the “hard law” that public health and human rights advocates have sought to clarify and interpret in flexible ways, it is important to examine the role of the WTO in greater detail. Clearly, agriculture, public health, education and trade have both social and economic dimensions. NGOs and developing countries have criticized the WTO as being insufficiently responsive to social needs. The question arises, to what extent should social policy be incorporated into the WTO? If so, how should it be incorporated? “While many accept the WTO essentially as currently constituted, others find it increasingly difficult to conceive of a multilateral trade regime confined exclusively to promoting economic efficiency through trade liberalization, in isolation from other values” (Stein, 2001: 508 n. 106). José Alvarez argues that one’s 9 TRIPS-plus refers to standards that are either more extensive than TRIPS standards or that eliminate options under TRIPS standards (Drahos, 2001: 793). 30 analysis of the WTO is dependent upon one’s perspectives on the relevant stakeholders and the WTO’s mandate. While some maintain that the WTO strictly exists to serve the “producers of goods,” others see the WTO as charged with serving “marginalized developing countries, NGOs and individuals” (2002: 154). Indeed, the question is how to balance the interests of these stakeholders within a trade framework. Ultimately, the questions of linking public health, agriculture, education, intellectual property, and trade are both normative and political. We can approach these issues more systematically by considering two analytic dimensions: the reasons behind linking trade and social policy and the mechanisms for doing so. Reasons for linking trade and/or public health, sustainable agriculture or education could be: (1) normative (because linkage is demanded by justice and fairness); (2) coherence (because a free trade regime would simply not make sense if [public health, education or sustainable agriculture are] ignored); (3) consequentialist (because free trade will adversely affect [public health, education or sustainable agriculture]; (4) strategic (because linking these issues in a creative package deals leads to more effective negotiations as to all); or (5) effectiveness (because the more effective WTO approach to dispute settlement can be usefully “borrowed” to the benefit of [public health, education or sustainable agriculture] (Alvarez, 2001: 12). A second dimension is the mechanisms for linking these issues. At least three institutional alternatives exist within the WTO for balancing competing policy goals. The first would be to interpret TRIPS flexibly in order to facilitate national solutions to balance conflicting goals; the second would be to apply TRIPS stringently to establish a common floor that all nations would have to meet so as to limit national interpretive discretion; and the third would be for the judicial panel to engage in judicial activism on a case-by-case basis, taking into account the broader policy landscape (e.g., WHO, UNAIDS, The United Nations Conference on Trade and Development (UNCTAD) (Shaffer, 2004a: 468). The first route would give developing countries more scope to tailor the TRIPS provisions to their specific needs. The second would rest ultimate authority in the intergovernmental political bargain struck in achieving TRIPS. This approach would leave it up to states to negotiate trade-offs between public health and trade. The third route, frequently advocated by legal scholars pushing non-market agendas, would empower WTO judicial panels to resolve value conflicts. Public health or agriculture or education and trade could be linked interpretatively in a top down manner whereby the WTO’s Appellate Body would interpret the meaning of the relevant laws (interpretive linkage). Advocates of a broader stakeholder approach for the WTO, such as Robert Howse, argue that “[r]ather than attempt once again to decide what is ‘in’ or ‘out of’ the WTO, we should try to mold the rules and their interpretation to structure the interaction of the trading regime with other powers and authorities, both domestic and international, in a legitimate manner” (Howse, 2002: 112). With this perspective, legitimacy would be the yardstick by which to measure the process and outcomes. As Gregory Shaffer suggests, “scholars of different ideological orientations tend to identify their ideological goals with particular institutions and thus tend to idealize those institutions. Power tends to disappear 31 within their preferred institutions” (2004b: page #). However, one must examine these institutions in their political context, and that invariably implicates power. For example while the WTO judicial “interpretive” approach may seem appealing on its face, both Shaffer and Jeffrey Dunoff have made compelling arguments against it. First, as Shaffer points out, structural asymmetries militate against extensive developing country participation in WTO litigation (2004a: 472-4). For example, in many cases the costs of litigating a WTO claim ($300 to 400 thousand in attorneys’ fees) are prohibitive. “The ‘haves’ come out ahead in litigation at the international level where legal expertise is highly specialized and expensive” (Shaffer, 2004b: page #). Dunoff has argued that based on the General Agreement on Trade and Tariffs (“GATT”)/WTO record, panels are more likely to decide cases in ways that militate against a non-market outcome (2001: 1007-11). This argument powerfully questions the assumption that judicial activism would tend in a “progressive” direction. Furthermore, “WTO judicial bodies decide . . . cases in a highlycharged political context. They are not free from political pressure, even if they do not expressly take it into account. They have their own institutional interests at stake” (Shaffer, 2004b). “The WTO Appellate Body operates not as an ideal neutral judge, but one that takes into account its own institutional interests and shapes decisions to encourage compliance and consensus” ”(Shaffer, 2004b; see also Smith, 2003: 74-80). Analysis of the Shrimp/Turtle case pertains to the prospects of the WTO’s representation of a broader range of stakeholders. While parallels between access to medicines, agriculture, educational materials and the Shrimp/Turtle case are inexact 10, the Shrimp/Turtle case seems to serve as a Rorschach ink blot test in legal scholarship. Scholarly interpretations of the significance of the case are hardly unambiguous. Some scholars advocating a broader stakeholder approach to trade find hope in the case, whereas others are far more wary. The first group of analysts argues that Shrimp/Turtle provides an important precedent for enlarging the WTO’s scope beyond trade. The second group bemoans the Shrimp/Turtle case for stretching the WTO too far. The third group draws more pessimistic conclusions about the significance of the case for WTO judicial activism. I will discuss each group in turn. Advocates for change and for expanding the WTO’s mandate conclude that the Appellate Body report in the Shrimp/Turtle Case “abandoned the WTO’s isolationism, that the WTO is a self-contained system . . . . by examining whether an endangered species was an exhaustible resource, by referring to international environmental law” (Gathii, 2001: 155). The Appellate Body referred to “a baseline in actual international environmental law that was contained in the Rio Declaration on Environment and Development” (Howse, 2002: 110). Howse cites the Beef Hormones and Asbestos cases as additional examples of the Appellate Body’s use of “a variety of jurisprudential techniques” to address the balance of economic and social values (2002: 109). James Thuo Gathii also notes that the Appellate Body endorsed Article 31 of the Vienna Convention on the Law of Treaties as an interpretive reference for the WTO in the Standards for Gasoline case. In his judgment, “such instructions come down to a ‘recognition that the General Agreement is not to be 10 E-mail from Gregory Shaffer, Nov. 23, 2003 (on file with author) 32 read in clinical isolation from public international law’” (Gathii, 2001: 156)11 Joost Pauwelyn argues that “WTO rules are not the alpha and omega of all possible trade relations between states. Other more detailed or special rules of international law . . . continue to be highly relevant” (2001: 540). These analysts advocate the fuller explicit incorporation of social issues into the WTO and tend to highlight normative and consequentialist arguments to support their position. Further, they advocate interpretive linkage by invoking WTO Appellate Body decisions in defense of their arguments and the possibilities for expanded linkage (Abbott, 2000: 64; Bloche, 2002: 833-4; Charnovitz, 2002: 211; Gathii, 2001: 155-7; Gathii, 2002: 316; Pauwelyn, 2001: 556-61; Howse and Mutua, 2000). Some authors argue that social policy is already deeply embedded in the WTO (Gathii, 2001: 138-9). For example, M. Gregg Bloche argues that in the WTO system the protection of health has become “a de facto interpretive principle when disputes arise over members’ treaty obligations” (2002: 825). According to Bloche, the Doha Declaration on TRIPS and Public Health “has interpretative weight under the Vienna Convention on the Law of Treaties, as either a ‘subsequent agreement between the parties regarding the interpretation’ of TRIPS or ‘subsequent practice in the application of the treaty which establishes the agreement of the parties regarding its interpretation’” (2002: 842, and nn. 96-97). Gathii agrees, arguing that “[w]hat the Doha Declaration . . . does as a matter of law is not insignificant. It mandates reading the TRIPS Agreement in light of its objectives and principles, thereby giving countries a legal basis in the Agreement itself to argue in favor of public policies” (2002: 305). Others argue that the WTO should be restricted to trade, period, (Bhagwati, 2002: 133-4; Steger, 2002: 145) and advocate an incrementalist, bottom-up approach. These analysts tend to highlight strategic reasons and advocate “negotiation linkage.” This group also presents a narrower conception of the WTO’s mandate (Steger, 2002: 137-40). At the end of the day, these analysts warn about the dangers of expanding the WTO’s mandate in the absence of a prior political consensus among the member states (Alvarez, 2002b: 4). They raise concerns about the WTO’s resource constraints (Bhagwati, 2002: 132) and the dangers of a damaging backlash if the WTO gets too far ahead of its membership (Alvarez, 2001: 15-17). Both Jagdish Bhagwati and Debra Steger are careful to define their sense of the WTO’s ultimate purpose. For Bhagwati, it is to promote “non-coercive trade” as a “mutually beneficial phenomenon” (2002: 127). By this yardstick he believes that TRIPS has no place in the WTO. Steger defines the WTO as an institution dedicated to promoting freer trade via the norm of nondiscrimination (and not fairness) (2002: 139). Bhagwati, unlike the first group of commentators, sharply criticizes the Shrimp/Turtle decision for precisely the reasons that the other commentators praised it. As he states: [I]t would be more prudent for it not to let earlier findings be replaced so drastically as in the shift from the Tuna/Dolphin to the Shrimp/Turtle decisions, which was doubtless influenced to some degree by the environmental lobbies of the North. Instead, such dramatic reversals or changes are better made in negotiations than in courts (2002: 133-34). Quoting WTO Appellate Body Reports on United States – Standards for Reformulated and Conventional Gasoline, May 20, 1996, 35 I.L.M. 603 (1996). 11 33 Bhagwati fears the explicit introduction of non-market criteria as opening a “Pandora’s box” (2002: 133) and favors a stricter compartmentalization of functions between various international organizations. He believes, for example, that labor standards should be addressed in the International Labor Organization (ILO) and not the WTO. Some see this as a potentially cynical position – to shunt sensitive issues off to venues in which “words don’t matter,” using less authoritative institutions as a safety valve to defuse controversy (Helfer, 2004: 56-57; Shaffer, 2001: 38). Steger also favors compartmentalization out of acute resource constraints as much as principles. For example, Douglas Irwin has pointed out that the WTO has a staff of only 500 (300 of whom are translators), and an annual budget of just $77 million. By contrast the World Bank employs 6,000 people and has an annual budget of about $8 billion, and even many NGOs have much bigger budgets than the WTO. The World Wildlife Fund’s annual budget is $360 million, and Greenpeace has a budget of about $120 million (Irwin, 2002: 186). According to Eric Stein, one must question: [W]hether the global commitment to free trade is strong enough to sustain a significant expansion of WTO competence to the full scope of trade-related environmental, social, and sustainable development issues. . . . For both the ‘nationalists’ concerned about state sovereignty, and the liberals concerned about democracy, the answer may be to ‘stop the integration’ and allow other (less integrated) international agencies (e.g., the ILO and mechanisms established by environmental treaties) to deal with the other values (2001: 507). These analysts trace the development of the GATT/WTO system as a “bottom-up” process (Alvarez, 2001: 19; Jackson, J., 2002: 125). As such, they recommend that its continued evolution rest on a political process of negotiation among member states. Ultimately, these authors see the problems as essentially political and therefore advocate political solutions. Insofar as the WTO is “a system of rules,” the “normative problems of interpreting and applying those rules . . . cannot be avoided” (Steger, 2002: 138). Steger takes direct aim at the analysts who champion the Appellate Body’s potential to expand WTO’s purview. As Steger states, “this challenge - of redefining and clarifying the values and policy objectives that the international community believes should trump the value of freer trade - is too big and too important to be left to the judicial branch of the WTO, even at its highest level, the Appellate Body” (2002: 144). A third group of scholars endorses a broader mandate for the WTO, that it should conform to a stakeholder model (Trebilcock and Howse, 1999: 54-56), but disagrees on mechanisms. As Dunoff states: Debates within and about the WTO tend to be consequentialist. That is, they tend to argue over what results will follow from adopting this or that rule, and whether such outcomes are desirable. In this context, the ‘desirable’ outcome is typically understood to be the outcome that maximizes economic welfare. But it is surely a mistake to understand the new trade issues exclusively in consequentialist terms (2001: 1008). Indeed, the new trade issues (such as intellectual property) are distributional and are not about “expanding the pie” (Dunoff, 2001: 1008). While Dunoff and Shaffer subscribe to 34 the broad stakeholder view, they part company with Howse and Gathii insofar as they are leery about a “top-down” Appellate Body incorporation mechanism. Scholars emphasizing the deeply political universe of the WTO draw different conclusions from the Shrimp/Turtle case (Dunoff, 2001: 984; Smith, 2003: 88-89). For instance, Dunoff argues that the Shrimp/Turtle case presents an ambiguous picture for champions of incorporation of “trade and” in the WTO (2001: 984-85). While heralded as a major step forward insofar as the Appellate Body held that “dispute resolution Panels and the [Appellate Body] itself have legal authority to receive amicus briefs and other materials from NGOs,” (Dunoff, 2001: 984-85) in fact the Appellate Body “proceeded to largely ignore the NGO arguments and instead ‘focus’ solely on the arguments the United States presented in its ‘main submission’” (Dunoff, 2001: 985). Surveying additional cases, Dunoff concludes that WTO trends are a step backwards for advocates of greater NGO involvement in WTO deliberations. Rather than representing the infusion of independent ideas into the deliberative process, NGO submissions are routinely ignored unless adopted by one of the parties to the dispute. In essence, this has had the effect of diluting the possibilities of NGO input. “While doctrinal developments deprive NGOs of a powerful rhetorical argument about the closed nature of WTO dispute resolution, the actual procedure used effectively excludes NGOs from WTO dispute resolution” (Dunoff, 2001: 987). INSTUTIONAL DIMENSIONS: PUBLIC INTERNATIONAL LEGAL REGIMES, INSTITUTIONS, HARD AND SOFT LAW: WIPO, FAO, CBD, UNHCHR, AND WHO The WTO is arguably the most important institution governing global intellectual property policy. However, TRIPS is not the only important public international legal regime covering global intellectual property, and the WTO is not the only significant venue. WIPO, the Food and Agriculture Organization (FAO), the Convention on Biological Diversity (CBD), the United Nations High Commissioner for Human Rights (UNHCHR), and the WHO are all actively engaged in making public international law in intellectual property. The following section seeks to map out the broader institutional terrain, focusing on WIPO, FAO, the CBD, the UNHCHR, and the WHO. As Stein points out, “any effort to rank [International Intergovernmental Organizations (IGOs)] in a hierarchical way is fraught with difficulties” (Stein, 2001: 495). However, it is important to highlight each organization’s perspective on the issues before discussing the relationships among them. This discussion of the institutional dimensions of global public policymaking requires exploring the reasons for shifting from one forum to another, the available options, the choice of institutions, and the role of the WTO. According to Helfer, there are four main reasons why actors strategically choose to shift forums: “to help achieve desired policy outcomes[;] to relieve political pressure for lawmaking in other international venues[;] to generate counter[-]regime norms[;] and to integrate those norms into the WTO and WIPO” (Helfer, 2004: 53). These reasons are not mutually exclusive. Of these four reasons, only the second, the “safety valve” strategy, is pursued in order to preserve the 35 status quo.12 “[S]tates and interest groups can use regime shifting as a safety valve, consigning an issue area to a venue where consequential outcomes and meaningful rule development are unlikely to occur” (Helfer, 2004: 56). The other three are pursued in order to effect change. Governments, private actors, and NGOs all engage in forum shifting. Forum shifting can be done horizontally, across institutions, as in the case in which the United States pushed to move intellectual property policymaking from WIPO to GATT for the Uruguay Round (Sell, 1998: 105). Forum shifting can also involve vertical moves across levels of governance, such as the United States’ use of Super 301 of U.S. trade laws to coerce developing countries into adopting higher standards of intellectual property protection, or its more recent efforts to use bilateral and regional intellectual property and investment treaties to secure “TRIPS-Plus” protection in developing countries (Drahos, 2001: 792-3). Actors may also choose among different institutions favoring those that afford them better access or those whose philosophies resonate more closely with their own goals. For example, actors can select a forum in which previously marginalized issues get a better reception. This can provide them with opportunities to propose and experiment with policy approaches to the issues (Helfer, 2004: 55). Forum shifting can provide governments that are critical of TRIPS a “safe space” in which to exchange information, develop soft law, and craft viable policy alternatives that address their concerns (Helfer, 2004: 58). Such soft law forums as the WHO and the CBD have proven to be significant incubators of alternative approaches, or “counter-regime norms,” to TRIPS. As Helfer suggests: [E]mbedded in the very idea of counterregime norms is a more strategic understanding of legal inconsistencies, one in which states consciously create conflicts as a way to subvert the prevailing legal landscape and provide fuel for renegotiating principles, norms, and rules to reflect their interests more accurately. . . . [D]eveloping countries and NGOs used precisely this strategic approach in seeking to integrate the new rules developed in biodiversity . . . public health, and human rights regimes into the WTO and WIPO. [Such forum shifting] can also function as an intermediate strategy that allows developing countries to generate the political groundwork necessary for new rounds of intellectual property lawmaking in the WTO and WIPO. When adopting this ‘integrationist’ strategy, developing countries use regime shifting to shore up support from hesitant allies, vet competing reform proposals, and generate common negotiating positions which they then introduce into the two organizations (2004: 59-60). This strategy can also support the development of competing discourses that can change the way parties read TRIPS and are willing to apply it.13 Furthermore, these competing discourses can challenge various domestic political bargains and integrate a broader range of viewpoints and parties into the issues. They can raise the political costs 12 This is the approach that the US is pursuing in WIPO in discussions of a development agenda. The US maintains that development issues do not belong in WIPO, and thus far is recommending that the discussions be sidelined onto a committee with a mandate only to discuss the issue. 13. E-mail from Gregory C. Shaffer, Professor of Law, University of Wisconsin Law School, to Susan K. Sell, November 23, 2003 (on file with author). 36 of defending the status quo (Sell and Prakash, 2004: 143-175). With this in mind, the following discussion surveys TRIPS-related activity in diverse forums. WORLD INTELLECTUAL PROPERTY ORGANIZATION The NIEO negotiations on the revision of the Paris Convention for the Protection of Industrial Property took place in WIPO, which administered the Paris Convention. In the mid-1980s, in the waning days of those stalled negotiations, dissatisfied American negotiators shifted intellectual property deliberations out of WIPO and into GATT. Americans, seeking high protectionist norms for intellectual property, favored GATT because it would permit them to link intellectual property protection to trade. The U.S. negotiators anticipated better results owing to the large and attractive U.S. market that could be used as negotiating leverage. The forum shifting of the mid-1980s was a major blow to WIPO’s morale and prestige. However, it has bounced back with renewed energy. Since then, WIPO has substantially transformed itself from a relatively sleepy, albeit highly competent, organization into a more entrepreneurial agency with a mission to prove its continued relevance to intellectual property owners. Unlike many international organizations, WIPO is almost self-sufficient. Rather than relying upon government handouts and grants, WIPO earns nearly ninety percent of its operating budget from its administration of the Patent Cooperation Treaty (PCT) (Doern, 1999: 111; WIPO, 2001). The biggest users of the PCT are the global corporations engaged in producing knowledge-intensive products and processes, such as the global life sciences industries and the financial services industries. These corporations also are the most ardent champions of high protectionist norms for intellectual property. These budgetary facts behind WIPO undoubtedly compromise its image as a technocratic, objective civil servant. The resurgence of WIPO troubles many supporters of a development agenda. Sisule Musungu notes that WIPO reflects “a build up of significant bias against latecomers to the intellectual property field” (2005: 4). WIPO is now regarded as being much more sympathetic to industry interests than it had been in the past. Furthermore, developing country WIPO delegates come from intellectual property offices that are socialized to favor protection (Drahos, 2002: 785); the professional camaraderie among intellectual property lawyers from both North and South means that discussions are far less adversarial than is the case in WTO.14 WIPO actively provides technical assistance to developing countries as they seek to comply with TRIPS. Those who pay WIPO’s freight undoubtedly shape its advocacy, and it has urged a number of developing countries to adopt “TRIPS-Plus” provisions in their One glaring example of this can be found in the WIPO publication entitled, “Striking a Balance: Patents and Access to Drugs and Health Care” at http://www.wipo.int/aboutip/en/studies/publications/health_care.htm in which WIPO, “despite the adoption of the Doha Declaration on the TRIPS Agreement and Public Health in November 2001. . . essentially labels all the concerns that developing countries have raised with regard to TRIPS and public health as ‘myths’ four years later” (Musungu, 2005: 7 and at note 43). 14 37 national legislation. For example, WIPO assisted in formulating the revised Bangui Agreement for the Organisation Africaine de la Propriete Intellectuelle (OAPI) countries. This agreement goes further than TRIPS by placing greater restrictions on the issuance of compulsory licenses and prohibiting parallel imports (Commission on Intellectual Property Rights, 2002). Indeed, in the wake of TRIPS, [t]he United States regularly sent lawyers for the U.S. pharmaceutical and copyright industries to Geneva as ‘faculty’ of the World Intellectual Property Organization. . . to teach developing country representatives about intellectual property matters and to draft ‘model’ laws for their consideration. Industry successfully lobbied Congress to allocate funds for these ‘educational’ efforts” (Shaffer, 2004a: 476). WIPO is also conducting negotiations on a Substantive Patent Law Treaty (SPLT) that aims for harmonization of patent law globally. After a failed effort that ended in 1991, new talks began in 2001. The U.S. and the EU also have been engaging in forum-shifting by taking some of their unmet concerns to WIPO (Musungu, 2005). This latter move is particularly intriguing given their audacious shifting of many intellectual property issues out of WIPO and into the GATT in 1986 at the outset of the Uruguay Round (Sell, 1998; Braithwaite and Drahos, 2000). At the time the U.S. expressed frustration that WIPO was too focused on North-South issues and sought an alternative forum in which the U.S. had more leverage. Industry leaders welcomed the intellectual property-trade linkage as being more effective in realizing their interest in higher standards of intellectual property protection. Now that intellectual property issues have received so much unwelcome attention in the WTO, the U.S. and EU have turned their attention to WIPO. Many suspect that the momentum behind the renewed effort is animated by a quest to increase property rights protection beyond that embodied in TRIPS and to universalize TRIPS-Plus standards. Developing countries have also seized opportunities to press their agendas, which are more fully developed in other venues, within WIPO. Developing countries have pressed for disclosure of origin and benefit sharing systems within WIPO. They sought to link biodiversity issues to the 1999 WIPO Patent Law Treaty negotiations by proposing the incorporation of the CBD recommendation that intellectual property applicants, when using genetic resources, prove that they had obtained informed prior consent to access those resources (Helfer, 2004: 62). In response, WIPO agreed to establish a separate body within WIPO to address intellectual property aspects of resources and traditional knowledge (South Centre and CIEL, 2005a: 16). Subsequently, the Intergovernmental Committee on Intellectual Property, Genetic Resources, Traditional Knowledge and Folklore (IGC) has conducted a number of studies that reflect the developing countries’ concerns as expressed in the CBD’s Conference of the Parties (COP). Developing countries have also requested that the WIPO Secretariat examine the implications of the SPLT for the IGC’s work, that “illustrates their increasing recognition of the need to coordinate lawmaking not only across different regimes or across venues . . . but also in different fora within the same intergovernmental organization” (Helfer, 2004: 71). 38 In 2004 a group of developing countries (Group of Friends for Development) proposed a development agenda for WIPO.15 The U.S. objected, arguing that WIPO is not a development agency but an organization specializing in intellectual property (Musungu, 2005: 4; WIPO document IIM/1/2). The General Assembly created an intergovernmental process to discuss the proposal, which met three times (in April, June and July 2005). At the end of the July meeting the group was unable to reach a consensus on continuing the process due to the opposition of the US and Japan (Musungu, 2005: 4). In closed informal meetings, delegations argued over whether discussions should continue in the high-level intergovernmental meetings that report directly to the General Assembly, or be consigned to a minor technical body, the Permanent Committee on Co-operation for Development Related to Intellectual Property (PCIPD) (ICTSD, 2005: 22). Those favoring high standards of intellectual property protection, such as the US, sought to confine discussions of “development” issues such as disclosure of origin and genetic resources to the IGC (presumably where “words don’t matter”). By contrast the US, Japan and the European Patent Office proposed high level discussions on issues of interest to them, such as definition of prior art, grace period, novelty, non-obviousness/inventive step (Correa, 2005b: 4, n. 14). In June 2005 the Friends of Development, led by Brazil, “expressly linked the development agenda to the Substantive Patent Law Treaty under elaboration at WIPO, refusing to discuss the latter in the absence of progress on the former” (ICTSD, 2005: 22). The group succeeded in halting progress on a SPLT, holding it hostage to meaningful, substantive progress on a development agenda. WIPO is institutionally constrained from pressing very far on issues that its main constituency (users of the PCT) opposes. WIPO has worked hard to get back into the good graces of the OECD since 1986, and it seems unlikely that WIPO would jeopardize its position by directly challenging the high protectionist agenda (May and Sell, 2006: 214). Indeed, playing on this very sensitivity, the U.S. delegate warned WIPO not to pursue a substantive development agenda; he said “we support WIPO. We would not want to change WIPO in a direction that would diminish that support” (IP-Watch, 2005. 11 April). A number of industry representatives, including former US Patent and Trademark Office official Bruce Lehman and civil society groups sympathetic to the US position issued a letter urging WIPO not to be diverted from its mission by a “so-called development agenda” (IP-Watch, 2005, 14 April). In June 2005 the U.S. PTO issued a press release bemoaning the impasse over the patent law harmonization talks at WIPO stating that, “the impasse also raises serious questions as to whether WIPO is even a viable forum for further meaningful patent discussions” (PTO, 2005). The Director of the Office of International Relations at the U.S. PTO, Lois Boland remarked that “’WIPO appears to be facing a serious identity crisis, underscoring the need to consider alternative approaches for achieving harmonization . . . Our users have spoken in no uncertain terms about their need for progress in this area’” (PTO, 2005). At the November 2005 WIPO General Assembly industry lobbyists showed up to express their opposition to a development agenda. As Argentina and Brazil presented the proposal to WIPO’s General Assembly. The proposal was co-sponsored by Bolivia, Cuba, the Dominican Republic, Ecuador, Egypt, Iran, Kenya, Peru, Sierra Leone, South Africa, Tanzania and Venezuela. WIPO document WO/GA/31/11 (September/October 2004). 15 39 reported in IP-Watch “one industry source said they were there for an ‘anti-development agenda’” (IP-Watch, 2005, 4 November). Eric Smith, head of the International Intellectual Property Alliance (a very powerful US-based copyright lobbying organization) stated that the development agenda was “revisionist” and that if its proponents “can convince the rest of the world to change it, good for them. But they won’t” (IP-Watch, 2005. 11 November 11). Nonetheless, WIPO held the first of two week-long meetings of the Provisional Committee on Proposals Related to a WIPO Development Agenda (PCDA) in late February 2006. The US is determined that WIPO’s mandate to promote intellectual property protection is not compromised by the development agenda. The US proposals included the following suggested topic: “counterfeiting and intellectual property: development’s antonym” (IP-Watch, 2006a, 24 February). Both China and Brazil argued that it did not belong on the agenda. Industry was out in force for these meetings. Director General of Brussels-based CropLife (a biotechnology and crop industry association), Christian Verschueren, expressed concern that “’the entire IP concept is under attack’” and that “the industry is being put into a defensive corner ... and the need for IP is no longer taken for granted” (IP-Watch, 2006b, 24 February). Jacques Gorlin, speaking on behalf of the ABIA, stated that “we think industry’s comparative advantage is telling a story. . . . The message will be: ‘We are the people who you are depending on to generate commercial benefits. If you do it through the disclosure system, it ain’t gonna happen’” (IP-Watch, 2006. 2 March). Gorlin was a key player in the origins of TRIPS as consultant to the Intellectual Property Committee (IPC). The IPC, made up of 12 CEOs of US-based global firms with large intellectual property portfolios, mobilized transnational private sector and governmental support for TRIPS and drafted major portions of TRIPS (Sell, 2003). Gorlin has now formed the ABIA to continue industry advocacy in multilateral, bilateral, and US government forums. Member companies include: Bristol Myers-Squibb, Eli Lilly, Hana Biosciences, General Electric, Merck, Pfizer, Procter & Gamble and Tethys Research (ABIA). At least half of these firms participated in the original IPC. ABIA is leading the lobbying fight to preserve and promote patents on life forms and is targeting activities at WIPO, WTO and CBD. The ABIA plans to lobby its allies, the US, Australia, Canada, Korea, Japan and New Zealand, as well as work with India’s biotechnology industry to try to soften India’s negotiating stance (IP-Watch, 2006, 2 March). At a side event that CropLife hosted during the WIPO meetings, deputy director general of WIPO Rita Hayes defended WIPO”s technical assistance programs and underscored the positive role that intellectual property protection plays in agriculture. She highlighted golden rice as “an example of a product that was IP-protected but licensed and available in a number of developing countries (containing vitamin A which helps prevent blindness in children)” (IP-Watch, 2006c, 24 February). Recall that patent thickets actually delayed the availability of golden rice for over a year and a half (Phillips, 2004: 181). Another side event during these talks addressed access to digital books. Panelists included industry and NGO representatives. Teresa Hackett of Electronic Information for 40 Libraries highlighted copyright-related obstacles to access to educational materials for libraries and suggested that license fees and rights clearance create major burdens for developing countries’ libraries (IP-Watch, 2006d, 24 February). Hackett expressed her support for the WIPO development agenda and a Chilean proposal for studies on the impact of IPRs on education in developing countries; “’Why not give developing countries the same flexibilities that developed countries had when they developed?’ Hackett said” (IP-Watch, 2006d, 24 February). FOOD AND AGRICULTURE ORGANIZATION The United Nations Food and Agriculture Organization (FAO) is charged with leading international efforts to defeat hunger. It focuses on developing rural areas, sharing policy expertise, and providing a neutral forum for states to negotiate agreements and debate policy (www.fao.org). In the early 1980s, “Cary Fowler, a former political activist who during the 1980s opposed the extension of IPRs to life-forms, decided that forumshifting was a game that the weak could also play. . . .Fowler and [Pat] Mooney identified the FAO as the most promising arena” (Dutfield, 2002: 14). Having honed their advocacy skills in battles over legislative activity in North America, they brought their campaign to the FAO. According to Fowler, the progressive extension of property rights with no commitment to conserving genetic diversity led activists such as himself and Pat Mooney to “develop a strategy and set it to work in a new but potentially friendlier arena” (1994: 180). The 1981 biennial conference of the FAO marked a shift in the discourse and strategy over plant genetic resources. Mooney and Fowler had consulted with the Mexican ambassador to the FAO prior to the meeting and had provided the Mexican president with reports on genetic resources. The ambassador arrived armed with proposals for the establishment of an international gene bank and an international legal convention for the exchange of genetic resources (Fowler, 1994: 181). Shifting to the FAO was a strategic move for two reasons: first, it shifted the center of gravity from American to developing country interests, and second, it broadened the discourse beyond patenting plant varieties to the broader connections between patenting, genetic conservation and development issues (Fowler, 1994: 181-2). As Fowler states, this campaign “show[ed] how new arenas can help radically alter power relationships, encouraging various responses from the disadvantaged – including a search for yet new arenas. The development of new technologies add[ed] fuel to the political fire by adding potential value to the botanical materials found in the Third World” (Fowler, 1994: 180). At issue was the one-way flow of germplasm from the genetically rich geopolitical South to the North (Kloppenbeurg, 2005). During the UN debates over a New International Economic Order (NIEO) in the 1970s developing countries and industrialized countries clashed over what constituted the “common heritage of mankind” (Sell, 1998). Industrialized countries considered landraces, or wild cultivars (a.k.a. folk varieties), to be the common heritage of mankind, ensuring free and easy access to germplasm from the South. However, they asserted property rights in industrialized countries’ breeders’ plant varieties. Raustiala and Victor have referred to this distinction as “raw” versus “worked” 41 germplasm (2003: 5). Indeed in the current discussions over Traditional Knowledge (TK), advocates for TK protection argue that what Northern plant breeders consider to be “raw” is in fact the product of on-farm innovations over generations. In 1983 negotiators agreed to the International Undertaking on Plant Genetic Resources (IUPGR) against the adamant opposition of the U.S., U.K., Australia and major seed companies. The non-binding undertaking was designed to ensure conservation and unrestricted availability and sustainable utilization of plant genetic resources for future generations by providing a flexible benefit and burden sharing framework (Dutfield, 2005: 15). Negotiators also established a new FAO commission on Plant Genetic Resources where states could discuss and monitor the non-binding undertaking. The US tried to boycott the Commission but 93 countries were represented at its first meeting in 1985. It is now the largest FAO Commission with 161 members and the EU (Dutfield, 2005: 15). Beginning in 1989 the IUPGR incorporated Pat Mooney’s notion of “farmers’ rights” “to acknowledge the contribution that farmers have made to conserving and developing plant genetic resources” (Dutfield, 2005: 15). The notion was to safeguard the rights of farmers to “work with, and live from, farming systems based on diversity, in the face of expanding monocultures and uniform seeds” (GRAIN, 2005). Developing countries sought to address the inequities arising from a system in which they lacked open access to improved varieties bred by seed companies, especially when some of those “worked” resources were based on developing countries’ “raw” germplasm (Raustiala and Victor, 2003: 12). Many developing countries perceive that the industrialized countries have profited from the existence of biological resources to the detriment of the countries in which they reside. The 1989 IUGR asserted the principle of unrestricted access and common heritage of both raw and worked germplasm. “The industrialized countries relied on the principle to continue their open access to raw PGR, yet refused to accept the undertaking’s principle of open access to worked PGR” (Raustiala and Victor, 2003: 13). Developing countries took a new tack in the late 1980s and began to see property rights in raw germplasm, “green gold” (Kuanpoth, 2006: 139), as a way to acquire wealth. Gene-rich countries could deny access to their biological riches and cut deals with companies that wanted to engage in bio-prospecting. Over the years developing countries have been abandoning the “common heritage” conceptions of biological resources and have asserted their sovereign rights to their exploitation. Access can be privatized effectively under the rubric of sovereignty and attendant rights of states over their own natural and cultural resources. Costa Rica’s nonprofit, private National Biodiversity Institute (INbio) struck a deal with Merck & Company to help underwrite INbio’s biodiversity prospecting plans (Reid et al., 1993). Once the Merck agreement was signed (September 1991) Costa Rica began to restrict access to its biological resources. Asserting property rights in biological resources has been controversial (for both worked and raw varieties). As Jack Kloppenburg has noted, it can be questioned: whether the whole farmers’ rights orientation was the proper way to go and whether there simply weren’t too many contradictions embedded in trying to use the master’s tools to dismantle the master’s house. . . . I think that the types of socalled alternative or community or traditional resource rights that have so far 42 been developed are really derivatives of Western intellectual property. . . . On the other hand, I don’t know what else can be done” (Kloppenburg, 2005). In any case, the 1991 Annex to the 1983 FAO Undertaking reflected this new approach and stated that “the concept of mankind’s heritage is subject to the sovereignty of states over their plant genetic resources” (quoted in Raustiala and Victor, 2003: 16). The 2001 FAO Treaty that came into force in 2004 has been sharply criticized as being a bonanza for plant breeders and a disaster for farmers’ rights. On the plus side, the FAO Treaty’s approach to access and benefit sharing does not follow the Merck-INbio model of bilateral contracting, but rather enables parties to: jointly create a multilateral system that gives everyone access on equal terms to the whole set of resources covered. . . . It recognizes that access itself is the main benefit to be shared, and aims to facilitate it rather than limit it by exclusive contracts and patents. Second, any monetary benefits generated through the system are to be pooled and used to support conservation and sustainable use efforts, rather than enrich any single provider” (GRAIN, 2005). While it falls well short of a generalized system of mutual access to all plant genetic resources for food and agriculture, it provides an alternative “that doesn’t lock all international seed exchanges into a tangle of bilateral contracts” (GRAIN, 2005). However, it clearly enshrines the notion of property rights over genetic resources. In the negotiations developed countries resisted any measures that would prevent their patenting of genetic resources, whereas developing countries wanted to limit the scope of the treaty to protect any business opportunities that might result from providing individual genes on the global market (GRAIN, 2005). In 2004 the FAO adopted a human rights and a food security frame for its Voluntary Guidelines on the Right to Food. 188 member states adopted the guidelines entitled “Voluntary Guidelines to Support the Progressive Realization of the Right to Food in the Context of National Food Security” (FAO, 2004). This means that states have pledged to work toward the goal of supporting the right to food. Advocacy groups supporting vulnerable populations endorse this approach because it focuses on the likely impact of trade policies and provides international mechanisms to hold economic actors accountable when domestic processes fail to promote or protect human rights (IATP, 2005: 5). Under international law, a human rights rubric provides mechanisms for states, groups and individuals to make their case (IATP, 2005: 5). CONVENTION ON BIOLOGICAL DIVERSITY The Convention on Biological Diversity (CBD) emerged out of the 1992 United Nations Environment Program’s “Earth Summit” in 1992. It was a response to growing alarm about species loss, the erosion of genetic diversity and the accelerating destruction of rainforests. The CBD is yet another international law that includes intellectual property elements. Unlike TRIPS and WIPO efforts, it more explicitly and squarely incorporates provisions that developing countries favor. The CBD recognizes the rights of indigenous 43 cultures to preserve their knowledge resources; Article 8(j) recognizes communal knowledge. The CBD conception challenges the TRIPS view that endorses the western, individualistic conception of knowledge ownership; this western perspective draws a sharp line between “folklore” and “science.” CBD stresses that biological resources are sovereign resources of states, whereas TRIPS enforces private property rights over them. Article 8j concerns the need to respect and preserve communal knowledge resources to ensure the sanctity of traditional practices and also “to promote their wider application with the approval and involvement of the holders of such knowledge.” Many developing countries and NGOs endorse CBD as a way of combating biopiracy in which global life sciences’ corporations expropriate genetic resources and traditional knowledge without authorization or compensation. W.R. Grace’s patenting of neem tree seed extracts became a lightning rod for this controversy. Furthermore, the CBD also offers more opportunities for upholding farmers’ rights against “biogopolists.” Article 8(j) calls for respect and preservation for “innovations and practices of indigenous and local communities embodying traditional lifestyles relevant for the conservation and sustainable use of biological diversity.”16 The CBD’s Conference of the Parties (COP), the CBD member states that decide how to apply and implement the CBD, has addressed the degree of the CBD’s compatibility with TRIPS. “After the entry into force of TRIPs, developing states led by China and the Group of 77 and sympathetic NGOs such as the World Wildlife Fund began to express concern over the relationship between intellectual property rights and the CBD’s access and benefit sharing rules” (Helfer, 2004: 33). The COP convened a panel of experts that led to the adoption of the Bonn Guidelines in 2002, which stipulated that applicants for intellectual property rights should disclose the origin of any genetic resources or related knowledge relevant to the subject matter. Such disclosures are meant to facilitate monitoring whether applicants have received prior informed consent of the country of origin and complied with the country’s conditions of access (Helfer, 2004: 29). While the CBD’s COP states have urged cooperation with the WTO and WIPO, they have: [P]ointedly refrained from ceding jurisdiction over biodiversity-related intellectual property issues to these organizations and instead are attempting to influence the terms of the debate by setting agendas, convening meetings, suggesting topics for further study, proposing a memorandum of understanding with WIPO ,and directing the CBD’s Executive Secretary to seek observer status with the TRIPS Council (Helfer, 2004: 34). India was the first to call for the primacy of the CBD over TRIPS Article 27.3(b) (the provision requiring members to provide protection for plant varieties either by patents or an effective sui generis system) and argued that TRIPS should be amended to comply with the CBD (Tejera, 1999: 981). Participants negotiating TRIPS agreed to revisit Article 27.3(b) four years after the date of entry into force (1999). Since 1998 the TRIPS Council has engaged in discussions to address the relationship between the CBD and TRIPS Article 27.3(b). Jonathan Hepburn has argued that the Article 27.3(b) review process in the TRIPS Council has been important in building developing country negotiators’ confidence and issue-specific knowledge (2002). At first discussions focused on the mandate of the review 16. Convention on Biological Diversity (“CBD”), June 5, 1992, art. 8(j), 31 I.L.M. 818, 825. 44 process, but broadened to include acknowledgment of diverse sui generis approaches to plant variety protection, concerns about indigenous peoples, and the relationship between TRIPS and the CBD (Hepburn, 2002). India, Brazil and the African group submitted papers to the TRIPS Council and delegations also began to acknowledge the relevance of the CBD and the international treaty of the FAO (Hepburn, 2002). Developing countries succeeded in incorporating these issues into the formal negotiating mandate of the WTO’s Doha Ministerial Declaration at Qatar in 2001. The WTO Ministerial Declaration of November 2001 instructed the TRIPS Council to examine the relationship between the TRIPS Agreement and the CBD (Abbott, 2002a: 489). This is an important development insofar as it constitutes a frank recognition of conflicts that need to be addressed. It also demonstrates the migration of an issue developed in the CBD into the WTO; the developing countries’ proposals were derived from the Bonn Guidelines (Helfer, 2004: 59-62). This is yet another instance of fomenting strategic inconsistency in order to bring conflicting values into sharp relief. The 2001 Doha Ministerial Declaration instructed the TRIPS Council to examine the relationship between TRIPS and the CBD. This relationship has yet to be resolved. Developing countries have been keen to amend TRIPS so that it will support the objectives of the CBD (South Centre and CIEL, 2005a:1). Seventeen countries have formed the Group of Like-Minded Megadiverse Countries, representing between 60 and 70 per cent of the biodiversity of the planet (South Centre and CIEL, 2005a: 2).17 So-called megadiverse countries want to halt unauthorized and uncompensated commercialization of their biological and traditional knowledge resources. They have pressed for TRIPS amendments that would require member states to disclose the source and country of origin of biological resources and traditional knowledge and to provide evidence of prior informed consent and benefit sharing. While states could adopt these policies in national patent systems, developing countries have argued that without an international agreement national level laws would do little to stop biopiracy (South Centre and CIEL, 2005a: 8). By contrast, the US prefers contract law to more global regulation. Alan Oxley, chairman of the Australian Asia-Pacific Economic Cooperation Study Centre, commented on his Centre’s PhRMAfunded study championing a free market approach and he argued that India and Brazil sought a “new regime of control” and “unnecessary regulation” (IP-Watch, 2005. 13 December). Susan Finston, Associate Vice President for Intellectual Property for PhRMA, and Executive Director of the ABIA, expressed objections to Peru’s efforts to regulate biopiracy and said that it had set up a “bio-piracy Gestapo” (IP-Watch, 2005. 15 December). Jacques Gorlin maintains that bio-piracy regulation mandating patent disclosure obligations in the Philippines, Colombia, Brazil and Peru have “dilute[d] property rights and . . . devalue[d] the genetic resources in the ground” (Gorlin, 2006: 4). Industry staunchly opposes any linkage between patents and benefit sharing and advocates a contractual approach to benefit sharing (ABIA; checkbiotech.org, 2006). While implementing a disclosure requirement would not stop all misappropriations of biological and traditional knowledge resources, it could help to improve the patent 17 Bolivia, Brazil, China. Colombia, Costa Rica, Democratic Republic of Congo, Ecuador, India, Indonesia, Kenya, Madagascar, Malaysia, Mexico, Peru, Philippines, South Africa, and Venezuela. 45 examination process regarding prior art. A September 2004 submission from Brazil, India, Pakistan, Peru, Thailand and Venezuela highlighted that beyond prior art, disclosure requirements would also “be useful in cases relating to challenges to patent grants or disputes on inventorship or entitlement to a claimed invention as well as infringement cases” (WTO, 2004, Brazil et al.: para. 5).This would help reduce the prevalence of patents that lack novelty or an inventive step. While not a panacea, a number of costly and timeconsuming cases of litigation could have been avoided with the disclosure requirements.18 Developing countries would prefer a binding requirement that contains noncompliance penalties through the WTO Dispute Settlement Mechanism, whereas the European Union has been sympathetic as long as “non-compliance with the requirement does not affect the validity or enforceability of the granted patent” (Correa, 2005: 6). This would seem to undercut the purpose of curtailing biopiracy. As Graham Dutfield argues: to be truly effective, the legal means should be made available to allow governments to challenge the patent’s legality in the jurisdiction in which it was granted if they consider a foreign patent they were not notified about may have been for an invention resulting from resources or traditional knowledge acquired under their ABS (access and benefit sharing) regulations (2005: 4). Therefore Dutfield recommends a higher standard than either voluntary or mandatory disclosure. A third way would be to require “proof of legal acquisition” to connect the patent system more strongly to the CBD’s access and benefit sharing provisions, especially for those countries directly providing the resources (Dutfield, 2005: 2). Dutfield argues that both mandatory disclosure and legal proof of acquisition might work very well in the context of pharmaceuticals. The non-generic pharmaceutical industry opposes mandatory disclosure (Smolders, 2005: 2) and has favored contractual arrangements for bio-prospecting such as the 1991 deal between Costa Rica’s INBIO and Merck (Reid, et. al. 1993). At the WTO Hong Kong Ministerial meeting in December 2005, Brazil, India and Peru aggressively negotiated to include provisions in the final Ministerial text exhorting the Director General to intensify consultations on outstanding implementation issues – specifically on the TRIPS-CBD relationship. India argued that without addressing the disclosure of origin of material in patent applications there would be no development package (IP-Watch, 2005. 15 December). The US, and global pharmaceutical and biotechnology companies strongly resisted agreeing to negotiate the disclosure issue (IPWatch, 2005. 17 December). Peru’s efforts to take a strong position on disclosure seemed to be ironic in light of the fact that it had just signed a bilateral deal with the US that incorporated the US’s preferred contractual approach to the issue. The US-Peru bilateral free trade agreement is TRIPS-Plus and endorses the utilization of contracts for protecting biodiversity. The US had been negotiating a broader Andean FTA but Colombia and Ecuador broke off 18 E.g., turmeric and camu camu, but not ayahuasca. See Correa, 2005: 3, IP-Watch 28/10/05. Also see Dutfield, 2005: 5-8 for a skeptical view. 46 negotiations in November 2005 over TRIPS-Plus provisions. The US-Peru FTA disclosure requirements appear in a “side letter” to the agreement stating that access to genetic resources and benefit sharing “can be adequately addressed through contracts” (IP-Watch, 2005. 17 December). Some sources at the WTO meeting argued that Peru had sold out and feared that the contract provisions meant that “companies could negotiate contracts with indigenous communities without any transparency and in this case without any requirements to disclose . . . from whom and where they obtained their resources” (IP Watch. 2005, 17 December). Furthermore the unequal bargaining power of the parties does not bode well for equitable deals. While Peru had been prominent in pushing for TRIPS-CBD linkage, critics blamed the US for its strategy of dividing coalitions through bilateral deals. The Hong Kong text calls for the WTO General Counsel to review progress and “take any appropriate action” by July 31, 2006 on implementation issues. The final text altered the earlier language urging the Director General to “continue with” his consultative process on implementation issues, such as CBD and geographical indications; the new text urges him to “intensify” his consultative process. The Indian Minister of Commerce and Industry, Kamal Nath, was pleased with the new language and with the deadline for review (IP-Watch, 2005, 18 December). The Europeans and the Swiss may attempt to link support for disclosure of origin to progress on geographical indications (South Centre and CIEL, 2004a: 10). The Europeans have been increasingly adamant about progress on geographical indications in the WTO (IP-Watch, October 28, 2005). The EU is seeking the establishment of a legally binding multilateral registration and notification system of geographical indications for wines and spirits (e.g. Champagne, Bordeaux) and the extension of protection of geographical indications for products other than wines and spirits (South Centre and CIEL, 2004a: 13). It appears that the supporters of the TRIPS-CBD linkage and geographical indications may join forces to achieve their desired outcomes. Swiss and EU proposals in WIPO have been sympathetic to developing countries’ concerns and may be designed to shore up developing countries’ support for geographical indications in WTO. The US opposes both positions and US industry has been particularly energized and vocal in its opposition. “Industry is concerned that the Swiss and EC proposals in WIPO may provide long-term legitimacy to those who seek to launch the WTO negotiations” (Gorlin, 2006: 4). HUMAN RIGHTS Human rights organizations increasingly have devoted their attention to intellectual property issues. Under a human rights rubric, intellectual property is recast as “a social product with a social function and not primarily as an economic relationship” (Chapman, 2002: 867). According to critics of the access campaigns: By advocating these human rights of access, IP skeptics seek to create a conflict with intellectual property rights, which give their owners the right to control and 47 exclude others. . . . Since advocates view “human rights obligations” as having “primacy” over economic policies and agreements, then it follows that intellectual property rights are secondary, to be treated as limited exceptions (Schultz and Walker, 2005: 84). Human rights organizations adopt resolutions, declarations, and reports that are not legally binding. “In July 2000, an NGO consortium composed of the Lutheran World Federation, Habitat International Coalition, and the International NGO Committee on Human Rights in Trade and Investment submitted a statement to the Chair of the Sub-Commission” on the Promotion and Protection of Human Rights (Helfer, 2004: 49). The statement underscored fundamental conflicts between TRIPS and human rights. In November 2000, the UN Committee on Economic, Social, and Cultural Rights held a day- long session on intellectual property rights, which led to the adoption of a statement in November 2001 endorsing a normative framework for intellectual property rights. The UN High Commissioner’s report on the impact of TRIPS on human rights addressed the medicines issue (UNHCHR, 2001). This report endorsed the public health and developing country activists’ position on TRIPS and highlighted the high cost of patented drugs as a barrier to health. It also discussed Brazil’s program as a positive model for expanding access to medicines. In this venue, NGOs, independent experts, and developing countries have framed the TRIPS rules as “a threat to economic, social, and cultural rights” and have displayed an antagonistic approach to TRIPS (Helfer, 2004: 46). The Sub-Commission on the Promotion and Protection of Human Rights has criticized the WTO quite sharply, stating in one draft report that “the WTO is a ‘veritable nightmare’ for certain sectors of humanity and criticize[d] WTO rules as ‘grossly unfair’” (Dunoff, 2001: 998). The WTO Secretariat responded by criticizing the report’s methodology, language, and conclusions, claiming that they were unsubstantiated by the evidence. The Secretariat suggested “a meeting with WTO officials to correct the ‘significant misunderstandings’ included in the report” (Dunoff, 2001: 999). The Sub-Commission has requested that the High Commissioner for Human Rights seek observer status with the WTO for the ongoing review of TRIPS (Chapman, 2002: 880). Patrick Wojahn points out that the right to health is guaranteed under numerous conventions including Article 12 of the International Covenant on Economic, Social and Cultural Rights (“ICESCR”), Article 25 of the Universal Declaration of Human Rights, and Article 11 of the Convention on the Elimination of All Forms of Discrimination Against Women (2001/2002: 466). Even though the United States has not ratified the ICESCR, Wojahn argues that the right to health should be considered to be “customary international law” because 143 states are parties to the Covenant and the right to health is included in numerous other treaties (2001/2002: 496). The UN human rights bodies have focused considerable attention on intellectual property issues, spanning public health, technology transfer, agriculture, indigenous peoples, and cultural dimensions of human rights. These bodies would like to see human rights concerns prevail over intellectual property rights. Many of the human rights approaches to health have been developed from the World Health Organization (Wojahn, 2001/2002: 469), again underscoring the migration of issues across institutions. This broad human rights rubric is strategic insofar as it “brings activists on narrower issues into the network of IP skeptics, for example, 48 medical groups with respect to patents and librarians with respect to copyright” (Schultz and Walker, 2005: 84). It thereby facilitates broader mobilization. WORLD HEALTH ORGANIZATION The WHO is a specialized agency of the UN system. Its mandate is to direct and coordinate authority for health work (Stein, 2001: 497). The WHO has the largest budget of all the specialized agencies, with an annual budget of “$1.8 billion dollars contributed by its 193 member states” (Volansky, 2002: 229). Since TRIPS, the WHO increasingly has been drawn into trade issues, and NGOs have had considerable access to the institution. In 1999, “the WHO … granted official status to nearly two hundred NGOs” (Stein, 2001: 498). Even though global pharma has an important voice in the WTO through its powerful OECD member states that contribute significant funding, the WHO has been criticized for its “failure to cooperate with the private sector” (Stein, 2001: 498). The director-general, secretariat, and health expert staff significantly shape agendas and outcomes. The WHO has been active in the access to essential medicines campaign (Sell, 2002: 504-06). Governments and NGOs first deliberated in the WHO over the very issues that led to the Doha Declaration on TRIPS and Public Health. In 1996, public health activists and developing country member states, including Brazil, South Africa, and Zimbabwe, expressed concerns about access to medicines in connection with globalization and TRIPS. The World Health Assembly adopted a resolution on a Revised Drug Strategy that asked the WHO to examine the impact of the WTO on national drug policies and essential drugs and to make recommendations for collaboration between the WTO and the WHO. “This resolution gave the WHO the mandate to publish, in 1998, the first guide with recommendations to Member States for implementing TRIPS while limiting the negative effects of higher levels of patent protection on drug availability” (‘t Hoen, 2002: 36). The WHO Essential Drugs Policy concentrates on the supply and use of about 250 drugs that are considered to be the most essential and important for public sector provision. In 1998, Zimbabwe’s Minister of Health asked Bas van der Heide of the NGO Health Action International (HAI) to produce a draft resolution for a “Revised Drug Strategy.” The Revised Drug Strategy is a document designed to assist developing countries in their health planning and policy implementation. The proposed document drew from work that HAI had been doing with consumer activist Ralph Nader’s group CPTech headed by James Love. Van der Heide and Love had crafted language for the Free Trade of the Americas negotiations advocating compulsory licensing and parallel importing, as well as stressing the priority of health concerns over commercial interests. “A small technical group within the WHO began to prepare and distribute concrete recommendations for coping with TRIPS by using the built-in flexibility to ameliorate the effects of introducing its requirements. These recommendations included . . . authorizing parallel importation and granting compulsory licenses where appropriate” (Abbott, 2002a: 474-75). This incensed the non-generic global pharmaceutical industry because the document endorsed the very practices that this sector was fighting through the USTR. 49 “The U.S. and a number of European countries unsuccessfully pressured the WHO in an attempt to prevent publication of the guide” (‘t Hoen, 2002: 36). Commercial pharmaceutical interests felt that the WHO’s involvement in this issue presented a threat to a trade-based approach. The United States has resisted the WHO’s efforts to help developing countries gain access to medicines, but the European Communities has shifted its views in recent years and has become more supportive of the WHO’s role in this area (Helfer, 2004: 42). In May 1999, the WHO’s World Health Assembly unanimously enacted resolution WHA 52.19 calling upon member states to ensure equitable access to essential drugs and review options under international agreements to safeguard access to these medicines (WHO, 2000). The WHO continues to pursue strategies designed to increase developing countries’ access to essential drugs. As Abbott points out: A number of training seminars regarding TRIPS implementation have been conducted with public health, patent office, and trade officials. These activities of WHO remain relatively unpublicized because increased attention would risk drawing a stronger reaction from Pharma. However, it is becoming substantially more difficult to find developing country officials who are unaware of compulsory licensing, parallel importation, and the importance of patent application reviews (2002a: 475). In 2001, the WHO adopted two resolutions that addressed the need to strengthen policies to increase access to medicines and the need to evaluate the impact of TRIPS (‘t Hoen, 2002: 36). It also published a bulletin highlighting the WHO’s policy guidelines and urging developing countries to refrain from implementing TRIPS-Plus intellectual property provisions (Helfer, 2004: 44; WHO, 2001). In the spring of 2001, the Zimbabwean Ambassador Boniface Chidyausiku requested a special TRIPS Council session on access to medicines. The session was held in June. The Quaker United Nations Office in Geneva provided support for developing country delegates, and a number of legal scholars, economists, and activists provided technical support (Sell, 2002: 512). As expressed by the Brazilian delegate, the meetings were intended to “eliminate the imprecision in international agreements concerning public health. In matters involving public health, developing countries wish WTO judges to interpret the TRIPS Agreement in a manner that benefits public health” (Viana, 2002: 314). The TRIPS Council resolved to continue analyzing the degree of flexibility afforded by TRIPS and planned future meetings on the issue. Momentum to address the issue accelerated throughout the summer and fall. The United States withdrew a WTO intellectual property case against Brazil, the UN General Assembly held a special session devoted to the HIV/AIDS pandemic, and UN Secretary-General Kofi Annan announced the establishment of a Global Fund to Fight AIDS, Tuberculosis, and Malaria (Sell, 2002: 513). In September 2001, the TRIPS Council met again to discuss the access to medicines issue. The African group presented a draft text for a ministerial declaration on TRIPS and public health, which emphasized that “[n]othing in the TRIPS Agreement shall prevent Members from taking measures to protect public health” (‘t Hoen, 2002: 39). 50 In the September preparations for the upcoming WTO Doha Ministerial meeting, some participants discussed the possibility of WHO/WTO collaboration in preparing a guide to assist developing countries in implementing TRIPS while protecting public health. An accidentally-leaked memo provided a WTO critique of WHO’s role (Abbott, 2002a: 475, n. 26). Australia mistakenly included an e-mail message from the then-Director of Intellectual Property for the WTO, Adrian Otten, in a submission to the TRIPS Council and then recalled it. The message states: [T]o be frank, I have my doubts about the wisdom and feasibility of attempting a joint guide with WHO and this still remains to be seen . . . . I do feel very strongly, for reasons indicated below, that we should not send it to WHO prior to Doha. .... I have two major concerns on the TRIPS side. The first and most important is that I think it unnecessarily risky for the WTO Secretariat to share texts on the TRIPS Agreement’s provisions on pharmaceuticals with the WHO at this stage . . . . It is important to recognize that there is a network which includes the leading nongovernmental people, certain people in the WHO Secretariat, . . . and many developing country delegates and nothing that is given to WHO can be relied upon to remain confidential. My second concern about TRIPS is that it does not, as yet, contain a section which discusses the positive impact of the TRIPS Agreement on public health, namely through promoting research and development into new drugs. .... The main messages that we would want to give are: (a) that open trade and a movement towards more open trade brings with it higher standards of health; and (b) concerns that the WTO rules will stand in the way of legitimate health measures 19 are unfounded. Not only does the message reveal a somewhat tense relationship between the two organizations, but it also clearly incorporates the PhRMA perspective as revealed in Otten’s “second concern.” Indeed, PhRMA executives have boasted about their close relationship with and extensive access to the WTO Secretariat (Basu, 2003; Reuters, 2003). In the run up to the November 2001 WTO Doha Ministerial meeting, human rights activists supported the WHO’s approach to the medicines issue (Chapman, 2002: 879) and participated in the access to medicines lobbying process. On September 11, 2001 terrorists attacked the United States using gas-filled passenger planes as bombs hitting the World Trade Center’s Twin Towers, the Pentagon and a field in Pennsylvania. The next month, some media and postal workers in the U.S. died from exposure to anthrax spores sent through the mail. In the shaken and uncertain climate of late 2001, American policymakers feared that the anthrax incidents heralded the launch of a major bio-terror attack. Both the Canadians and Americans threatened to issue compulsory licenses to seize Bayer’s patents on Cipro to ensure adequate stockpiles of the most effective drug for treating anthrax. Ultimately neither Canada nor the U.S. followed through on the threat, but just as Brazil had done in the past, they both negotiated deep price discounts with Bayer. 19. Posting of Mike Palmedo, mpalmedo@cptech.org, to IP-Health Listserv, Adrian Otten Missive on WTO/WHO Cooperation, at http://lists.essential.org/pipermail/ip-health/2001-September/001900.html (Sept. 21, 2001). 51 On the eve of the November 2001 Doha meeting, everyone was well aware of the irony that the U.S. had done precisely that for which it had criticized the Brazilians and harassed the South Africans. Led by the African group and Brazil, a coalition of developing countries stuck together and insisted that without clarification of the public health flexibilities in TRIPS and reassurances of immunity from prosecution there would be nothing to discuss at Doha. Their threat to walk away from the negotiations was credible, and since the developed countries were so eager to launch a new more liberalizing round the parties quickly reached agreement on the Doha Declaration on TRIPS and Public Health (Odell and Sell, 2006). While various stakeholders had different assessments of the significance of this declaration, developing countries saw it as a significant victory for their position that TRIPS must not stand in the way of providing medicines in public health emergencies (Sell 2003: 161-162). While not, by its terms, legally binding, it largely embraced the WHO and NGO view that TRIPS should not be a barrier to developing countries seeking access to medicines. This opens the possibility that the norms expressed in the Doha Declaration could become legally binding either through a dispute resolution report that so holds or otherwise.20 The African group and its supporters also sought clarification that nothing in TRIPS should prevent countries from exporting generic drugs to poor countries, but the Declaration postponed the politically contentious issue of the ability of generic manufacturers to export drugs to countries without manufacturing capacity. This was deferred to the so-called Paragraph 6 negotiations at the TRIPS Council. The WTO deadline for resolving that issue came and went in December 2002. The United States, reflecting the wishes of its brand name pharmaceutical corporations, stood alone and used its veto to block the interpretation that 21 140 other countries had supported. Meanwhile, the WHO busily tried to craft constructive approaches to this issue. A May 2003 WHO report endorsed the NGO/developing country approaches to the medicines issue (WHO, 2003). The report emphasized the neglect of tropical diseases, the Doha Declaration’s recognition that pharmaceutical products require special treatment, and the negative effects of patent protection on drug pricing. Further, the report recommended expanded competition as the most effective way to reduce drug prices. The report also took a critical view of “TRIPS-plus” provisions as being detrimental to health care. The director-elect of the WHO, Lee Jong-wook, announced measures that will make Brazil’s AIDS policy the foundation for the WHO efforts in this area. He asked the Brazilian Health Minister to release Paulo Teixeira, head of the administration’s AIDS program, “to formulate the new policy for combating AIDS throughout the world, based on Brazil’s 22 experience.” This represented important recognition of Brazil’s leadership role and support for the developing countries’ and NGO positions. 20.E-mail from Professor Jeffrey Dunoff, Professor of Law, Temple University, Beasley School of Law, to Susan K. Sell, November 13, 2003 (on file with author). 21. Larry Elliott & Charlotte Denny, U.S. Wrecks Cheap Drugs Deal, THE GUARDIAN, Dec. 21, 2002 (characterizing pharmaceutical companies’ intense lobbying efforts and Vice President Cheney’s intervention in talks as instrumental in U.S. refusal to relax global patent laws), available at http://www.guardian.co.uk/international/story/0,3604,864071,00.html. 22. Posting of Mike Palmedo, mpalmedo@cptech.org, to IP-Health Listserv, WHO to Adopt Brazilian Model to Fight AIDS/HIV, FIN. TIMES LTD., May 21, 2003, available at http://lists.essential.org/pipermail/ip-health/2003-May/004779.html. 52 The May 2003 World Health Assembly meeting on improving access to essential medicines was particularly volatile. The United States presented a resolution that neglected even to mention the Doha Declaration and did little more than assert the value of strong intellectual property protection as a stimulus for innovation.23 The U.S. proposal further requested the WHO to refer member states to the WTO and WIPO for assistance in implementing TRIPS obligations.24 Brazil proposed a resolution, supported by Bolivia, Ecuador, Indonesia, Peru, Venezuela, and South Africa on behalf of the members of the WHO African Region. The Brazilian proposal reflected developing countries’ concerns about access to medicines and called for an independent commission to examine the relationship between intellectual property rights, innovation, public goods, and public health. The developing countries sought an international committee much like the UK Commission on Intellectual Property Rights,25 which was critical of overly strong patent rights as a barrier to access (CIPR, 2002: 22). When it was clear that no one supported the U.S. resolution, the Brazilian, American, and several African delegations worked out a compromise that a WHO committee adopted by consensus. The resolution called for the establishment of a time-limited independent commission, and it omitted any reference to “TRIPS-Plus” obligations in bilateral and regional trade agreements. NGOs bemoaned the fact that the developing countries’ proposals had been watered down in the compromise. However, the Doha Declaration was prominently featured in the resolution, and Member States were urged to arrive at a solution to the Paragraph 6 Doha Declaration impasse prior to the Cancun WTO Ministerial in September 2003 (WHO, 2003, May 28). A TRIPS Council meeting held June 4th through 6th, 2003 ended in a deadlock over the Paragraph 6 issue. Harvey Bale, President of the Geneva-based International Federation of Pharmaceutical Manufacturers Associations, stated that there had been no progress since the talks collapsed in December 2002. He referred to the December sixteenth draft text as “a license to steal” and claimed that “all research-based companies 26 have problems with December 16.” However, just before the Cancun Ministerial in September 2003, developing countries threatened to hold the Round hostage in the absence of a Paragraph 6 agreement. The United States finally relented in its adamant opposition to developing countries’ proposals for a Paragraph 6 solution. The deal authorized any member state lacking sufficient pharmaceutical manufacturing capacity to import necessary medicines from any other member state. This waiver of TRIPS Article 31(f) (restricting compulsory licensing only to supply one’s domestic market) included procedural safeguards to prevent diversion of cheap medicines to rich countries’ markets (WTO Council on TRIPS 2003; Matthews, 2004a). Therefore according to the August 30th deal, generic copies of drugs made under compulsory license can be exported to countries lacking production capacity (WTO, 2003). 23. Posting of Nathan Ford, Nathan.FORD@london.msf.org, to IP-Health Listserv, Sparks Fly Over Patents and Vital Drugs at World Health Assembly, LANCET, May 31, 2003, available at http://lists.essential.org/pipermail/ip-health/2003-May/004816.html. 24. Posting of Cecilia Oh, ceciliaoh@yahoo.com, to IP-Health Listserv, Third World Network Info. Service, WHO Adopts Resolution on IPRs and Public Health After Wrangling Over Text, THIRD WORLD NETWORK, May 29, 2003, available at http://lists.essential.org/pipermail/iphealth/2003-May/004815.html. 25. Id. 26. Posting of Mike Palmedo, mpalmedo@cptech.org, to IP-Health Listserv, Richard Waddington, No Shift on Drugs at Trade Talks – Industry Chief, REUTERS, May 22, 2002, available at http://lists.essential.org/pipermail/ip-health/2003-May/004773.html. 53 The August 30th 2003 decision also included a Chairman’s Statement, emphasizing the “Members’ ‘shared understanding’ that the Decision will be interpreted and implemented on a ‘good faith’ basis in order to deal with public health problems and not for industrial or commercial policy objectives” and their agreement to take steps to prevent drug diversion to third markets (Matthews, 2004b: 11). According to James Love, Director of CPTech, the Chairman’s Statement was approved by Pfizer CEO Hank McKinnell and the office of Karl Rove, President Bush’s Deputy Chief of Staff in charge of policy (Love, 2005). The Decision also includes a list of 23 countries that agreed to opt out of the system as importers, 11 awaiting accession to the European Union pledging to use the waiver only in cases of “extreme emergency” until such time as they gain membership and will then opt out completely, and finally a group of 13 additional countries that pledged only to use the waiver in cases of extreme emergency (WTO, WT/GC/M/82, 13 November 2003: p. 7, para. 29). Many of these countries are concerned about whether they can “opt back in” in the event of an avian flu pandemic (IP-Watch, 25 October 2005). In the US, Senator Charles Schumer called for the compulsory licensing of Roche’s Tamiflu to ensure adequate supplies (Ip-health, 2005, 18 October, Message 15). By the end of the Doha negotiations, member states needed to decide upon the permanent status of the Paragraph 6 agreement on compulsory licensing and access to medicines. The August 30th 2003 Decision was a temporary measure pending final agreement. The questions that dominated the negotiations over Paragraph 6 on the eve of December 2005 the WTO Hong Kong Ministerial meeting were: 1) Should the August 30 th decision be included in Article 31 of TRIPS?; 2) What is the relationship between the Statement of the Chairman of the General Counsel and the August 30th decision? What is its legal status? The African group of countries proposed that the August 30th Decision be incorporated into Article 31 as an amendment to TRIPS, but recommended that both the Chairman’s Statement and the annex of the Decision that details “best practices” regarding packaging and manufacturing drugs to prevent diversion of low-cost medicines to highincome markets be excluded. The African Group argued that the Chairman’s Statement should not be part of the amendment as it was not part of the August 30th Decision, and opposed any elevation of the statement’s legal status (WTO, 2005, IP/C/W/440). The African group suggested that certain measures recommended in the annex on drug diversion would be costly (Matthews, 2004b: 7). The African group’s proposal received “a fairly hostile response from developed countries” (South Centre and CIEL: 2004a: 5). In 2004 Mozambique, Zimbabwe, and Zambia issued compulsory licenses for selected antiretroviral drugs, which helped to underscore the African group’s desire for an enduring solution (South Centre and CIEL, 2004a: 11). Kenya had tried to issue compulsory licenses for HIV/AIDS drugs under the waiver, but two European pharmaceutical companies holding the patents stopped them and offered voluntary licenses instead (IP-Watch, 2005. 6 December). The EU and the United States have insisted that the Chairman’s Statement and the annex be retained in any amendment. This reflects their concerns about drug diversion. Informal discussions between the African Group, the US, and the European Community (with the TRIPS Council Chair, Ambassador Choi Hyuck of Korea present) angered a number of developing countries. Argentina, Brazil and India protested their 54 exclusion from and the lack of transparency in these deliberations (IP-Health, 2005, Oct.28). Ultimately the African Group and the United States worked out a compromise. In the end, delegates voted to adopt the waiver as an amendment to TRIPS that includes Article 31bis, the waiver, one annex on terms and conditions, and an appendix on the assessment of pharmaceutical manufacturing capabilities (IP-Watch, 2005, 6 December, 10:53pm). In a rather elaborate compromise arrangement (“choreography”) the Chairman’s Statement was read aloud and adopted, and developing countries that in 2003 had agreed to opt out of the waiver except in cases of national emergencies had to restate their commitment. During the process no one was allowed to embellish his or her remarks in any way. Even though the final agreement did not mirror the original African Group proposal, a number of African delegations were pleased with the outcome. One delegate expressed relief that the uncertainty generated by the waiver was resolved as it is now a permanent part of TRIPS (IP-Watch, 2005 6 December 12:36am). Another developing country delegate said that “political pressure” had led the Africans to soften their stance and that “the deal was a matter of ‘not losing what you already have’” (IP-Watch, 2005, 6 December, 12:36am). While the US did not succeed in having the Chairman’s Statement incorporated as a footnote to the text (which would have elevated its legal status), USTR Rob Portman hailed the agreement as a “landmark achievement” and took credit for making it happen (IP-Watch, 2005, 6 December, 10:53pm). Non-generic pharmaceutical companies “welcomed the agreement to maintain ‘anti-diversion measures and other aspects of the ‘chairman’s statement’” (IP-Watch, 2005, 7 December). As a number of public health activists and NGOs immediately stressed, the amendment is disappointing for those advocating easy access to generic medicines. It comes with numerous conditions that make it difficult to use; this is one reason why not one country has availed itself of the August 2003 waiver (Doctors Without Borders, 2005, December 10). CPTech Director James Love stated that “big pharmaceutical companies and the EU” bullied developing country negotiators into accepting the deal; he referred to this “awful decision” as being “anti-consumer, anti-competition and anti-free trade” (CPTech, 2005. 6 December). Many NGOs suspected that developing countries were pressured into the deal so that WTO members would have something to show after four years of talks; they further presumed that the US and EU were desperate for a deal to “deflect attention from their lack of movement in agriculture and their anti-development proposals in NAMA and Services” (CPTech, 2005, 3 December). Additionally, Members agreed to grant Least Developed countries an extension until July 2013 before they are required to comply with TRIPS. Zambia had proposed an extension through 2020 against the US, Japanese and Swiss preference for reviewing extensions on a case-by-case basis. The 2013 deadline represents a compromise between these two approaches. In the meantime, least developed countries may not roll back any existing intellectual property laws unless they are already more stringent than TRIPS. Significantly, this decision came about one week before the December 6th TRIPS amendment agreement. According to one proponent of high standards of IP protection this was a strategic move designed to provide some results to make the WTO process seem 55 valuable, especially given the fact that no one expected movement on agriculture. Furthermore, he maintains that this decision took “a lot of hot air out of the activists (sic) balloon” and lessened “the risk of a disastrous weakening of TRIPS in Hong Kong” (IP Blog, 2005, 29 November). The WHO also acknowledged the relevance of human rights and public health in late January 2006. The WHO’s Executive Board submitted to the World Health Assembly (its highest decision making body) a resolution to consider a “Global Framework on Essential Health Research and Development”. Brazil and Kenya supported the resolution, which recognized the potential for public sector and open source methods of conducting medical research and development (ip-health; 2006, Feb. 24). CONCLUSIONS Even without obvious economic power, the African block, Brazil, India, and their NGO advocates have begun to make inroads on global intellectual property policy by strategically shifting forums and advancing new arguments. For instance, the CBD recasts intellectual property as both an environmental issue and a potential obstacle to sustainable agriculture. Environmental preservation and the ability of states to feed their own people are powerful values that can lead people to question the primacy of economic efficiency as the sole yardstick by which to measure policy effectiveness. Redefining intellectual property from being a trade issue to a public health issue resulted in the Doha Declaration. This has opened up an important space for debate on the costs and benefits of intellectual property protection as enshrined in TRIPS. Recasting intellectual property as a human rights issue could be an effective strategy to the extent that intellectual property rights are implicated in battles over the rights to food, medicines and educational materials. No single “reframing” alone will open up the dialogue and change global policies, but cumulative concerted efforts from a variety of angles could to a more balanced approach to intellectual property rights Soft and hard law developed in non-WTO venues via developing countries’ strategic forum shifting has enhanced their bargaining power within the WTO and WIPO. Developing countries and their NGO supporters deliberately promoted issue migration, on health from the WHO to the WTO, and on human rights and indigenous people to WIPO: [Strategic forum shifting] facilitates a proactive negotiating strategy, enabling governments and NGOs to coordinate their efforts around hard and soft law proposals first vetted and refined in other international venues. This integrationist approach also allows states to justify their demands for reform by invoking rules and principles endorsed by officials of intergovernmental organizations and by legal and technical experts. Support from these seemingly neutral actors gives the demands the imprimatur of legitimacy. And it allows proponents to frame their arguments as rational efforts to harmonize potentially inconsistent treaty obligations and soft law standards that many states have agreed to, rather than as self-interested attempts to distort trade rules or to free ride on foreign creators or inventors. Seen from this perspective, even the soft law intellectual property 56 standards generated in the biodiversity . . . public health, and human rights regimes have hard-edged consequences. They act as progenitors of proposals to revise legally binding rules within the WTO and WIPO (Helfer, 2004: 61). Returning to issues raised at the outset, it is important to bear in mind the relationships among international institutions. Given the fact that the WTO embodies hard law, it exists in somewhat of a hierarchical relationship compared to its soft law counterparts. Beyond the hard/soft law distinction, the WTO has cooperated more extensively with those international institutions (IMF and World Bank) that share its basic pro-market philosophy (Dunoff, 2001: 999). Indeed, the WTO also has expressed some overt antagonism toward both the WHO and the UNHCHR. International organizations involved in intellectual property issues are divided over the merits of diverse multilateral approaches to intellectual property protection. The WTO and WIPO seem to champion the interests of property holders over property users (or producers over consumers), whereas the CBD, the FAO, the human rights organizations, and the WHO promote approaches that at the very least seek to balance the rights of producers and consumers. These divisions provide opportunities for developing countries and NGOs to change the agenda by reframing issues in ways that enlarge their scope of action. While the WTO and WIPO seem to represent both the economically and politically most powerful, the work of the WHO, the FAO and the CBD can have an impact on the work of these other agencies. Nonetheless, since much of the debate over TRIPS interpretations will be discursive (Shaffer, 2004a: 476), “soft law will be an important tool for WTO panels to use in resolving . . . arguments [over competing objectives]” (Helfer, 2004: 77). While the deliberate generation of counter-regime norms in alternative forums has many benefits, it also risks the injection of further uncertainty and incoherence into efforts at global governance of intellectual property (Helfer, 2004: 75). Without authoritative guidelines for resolving such issues, it may facilitate outcomes that favor the structurally powerful at the expense of others. OBSTACLES Focusing on TRIPS and the letter of the law, one may conclude, as does Helfer, that states implementing their TRIPS requirements in good faith are unlikely to have their laws challenged successfully (2002: 34). However, public international law such as TRIPS is embedded in a broader context of asymmetrical power relationships between developed and developing countries, and between producers and consumers of the fruits of intellectual property. This context reduces the amount of leeway that poor states have in devising regulatory approaches that are most suitable for their individual needs and stages of development. One of the most important assets for developing country negotiators in the WTO is peripheral vision to stay abreast of the proliferation of intellectual property policymaking in diverse institutional settings. The US and the EU have been able to exploit resource disparities and shift forums whenever it suits their interests. This holds true of the shift from WIPO to WTO and back again, as well as the shifting between multilateral, bilateral 57 and regional negotiations. The biggest threat to any gains that developing countries may bargain for, or even achieve, in the Doha round of trade negotiations lies outside of the WTO. At the end of the Uruguay Round negotiators did not share consensual assessments of TRIPS. Negotiators from the United States and the European Union tended to see TRIPS as a floor – a minimum baseline for intellectual property protection. By contrast, developing country negotiators saw it more as a ceiling – a maximum standard of protection beyond which they were unwilling and/or unable to go. Given this perspective, it should come as no surprise that the U.S. and the EU aggressively have been pursuing efforts to ratchet up TRIPS standards, to eliminate TRIPS flexibilities and close TRIPS loopholes. Playing a multi-level, multi-forum governance game, countries like the United States have been able to extract a high price from economically more vulnerable parties eager to gain access to large, affluent markets (Abbott, 2005: 350-354; Correa, 2004; Vivas-Eugui, 2003). Bilateral Investment Treaties, Bilateral Intellectual Property Agreements, and regional Free Trade Agreements concluded between the U.S. and developing countries, and between the Europe Union and developing countries invariably have been TRIPS-Plus (Drahos, 2001; Dutfield, 2003b). For example in the intellectual property provisions covering agriculture in these agreements, developing countries are most often required to ratify or accede to UPOV91 as their sui generis system of protection, and “to undertake ‘all reasonable efforts’ to make patent protection available for plants” (South Centre/CIEL, 2004: 12). In recent years developing countries have begun to challenge this discrepancy between the multilateral rules and the TRIPS-Plus standards proposed in regional and bilateral agreements. In late 2005, Ecuador and Colombia broke off talks with the US over TRIPS-Plus issues and had refused to agree to TRIPS-Plus standards. However, in late February 2006 the US and Colombia concluded an agreement that includes TRIPS-Plus standards despite the best efforts of some Colombian negotiators to counteract them (USTR, 2006; Ip-Watch, 2006e). In Russia’s simultaneous negotiations for its accession to the WTO as well as for a bilateral deal with the United States, Russia’s lead negotiator on WTO accession, Maxim Medvedkov, has endorsed TRIPS but has balked at the TRIPSPlus demands. He stated that “I think we have to draw a line between WTO and bilateral issues” (IP-Watch, 2005. 24 October). This reflects Russia’s view of TRIPS as a ceiling and not a floor. According to Peter Drahos the US and its IP activist industries have been engaged in a “one-way ratchet” for intellectual property systematically obtaining higher levels of protection (Drahos, 2004: 55-61). As the International Chamber of Commerce points out, “the chain of national intellectual property laws will only be as strong as its weakest link, and the ability to meaningfully enforce rights will be crucial” (ICC, 2005: 13). Nothing in TRIPS prevents states from adopting stronger forms of protection, and the US and its industries increasingly are coordinating enforcement through a number of venues. Industry representation in the USTR advisory committees (IFAC-3 and IFAC-15), overlapping memberships in industry associations such as the Business Software Alliance (BSA) and the International Intellectual Property Alliance (IIPA), and ad hoc mobilization vehicles such as ABIA increase the information exchange among private actors and the USTR to 58 monitor compliance, negotiate and enforce TRIPS-Plus deals and lobby at national and multilateral levels. For example, Microsoft is a member of the IIPA, BSA and IFAC-3 (Drahos, 2004, 69). This thick network has resulted in a centralized system of private governance that enlists the USTR for legitimation and enforcement and heightens opportunities for rent-seeking (Drahos, 2004: 77). A particularly pernicious example of this is the Gleevec case in South Korea. Gleevec is a leukemia drug that was developed with assistance from the US Orphan Drug Act, under which the US government paid for 50% of the private sector costs of clinical trials (Ip-health, 2001). Swiss drug maker Novartis owns the patent. The drug costs roughly $27,000 per year per patient in the U.S., keeping it out of reach of most. In late 2001 Novartis suspended supply of Gleevec to South Korea because Novartis failed to get the price it sought from the South Korean government. The US, Swiss and Japanese had accepted the price of US$19.50 per pill27 during the Novartis-South Korean negotiations. Novartis directly approached Korean leukemia patients offering them a co-payment exemption if they would convince the South Korean government to accept that price. The patients refused. Rather than negotiating a lower price, the South Korean government sought to contain costs by excluding chronic phase chronic myelogenic leukemia (CML) patients from insurance coverage. Hae-joo Chung, Director of Equipharm project, issued a plea on behalf of the People’s Health Coalition for Equitable Society for global consumer and health groups to endorse its quest to get the South Korean government to restart negotiations with Novartis and resume supply – even if meant resorting to compulsory licensing in line with the Doha Declaration on TRIPS and Public Health (Ip-health, 2001). These health groups appealed to the Korean Intellectual Property Office and requested adjudication for the grant of a non-exclusive license to import generic Gleevec from India for the public interest because Korean CML patients were imperiled by unstable supplies and high prices (Ip-health, 2003). While Novartis is a Swiss company, the USTR supported Novartis in this case. Facing declining profitability in the European market, makers of potentially high profit drugs like Gleevec are turning to emerging middle income markets in Asia and Latin America to make up the difference (Benvenisti and Downs, 2004). In order to ensure the success of this strategy they must fend off generic challengers in these markets. As Benevisti and Downs suggest, the USTR intervened on behalf of Novartis in order to “prevent a precedent that might eventually damage the profitability of products manufactured by its own firms” (2004). Indeed, the Korean decision to reject the generic importation option under compulsory license incorporated the very language that USTR Robert Zoellick had been promoting in his efforts to limit the scope of the Doha Declaration on TRIPS and Public Health. The Korean government denied the petition on the grounds that CML was neither “infectious” nor likely to cause “an extremely dangerous situation in our nation” (Ip-health, 2003; Abbott, 2005: 328-336). As James Love of CpTech remarked, “’the U.S. government does not control the price of drugs in its own country but it is telling Korea what they should charge’” (quoted in Benevisti and Downs, 2004). This example highlights the intrusive reach of what Drahos calls the “nodal enforcement pyramid” that global IP-based firms and their governments deploy 27 Daily dosages range from 4 to 8 pills a day. 59 (2004). Asymmetrical power relations and the political influence of global high-technology industries continue to shape intellectual property policy. Given the expansion of intellectual property rights and unequal distribution of economic and political power across the globe, developing countries face new challenges in navigating the system to their benefit. OPPORTUNITIES The foregoing has important implications for the various access campaigns and intellectual property governance more generally. The access to medicines campaign, in particular, has provided several important lessons. Reframing issues can be an effective way of creating space for debate and reconsideration of the conventional wisdom. By recasting intellectual property as a public health issue, policy makers increasingly are forced to confront the unconscionable trade off between economic gain and unnecessary death. This has raised the political costs for those seeking to defend the status quo. “In a world of asymmetric power, developing countries enhance the prospects of their success if other U.S. and European constituencies offset the pharmaceutical industry’s pressure on U.S. and European trade authorities to aggressively advance industry interests” (Shaffer, 2004a: 450). When brought to light through public action and media attention, the use of economic coercion to reduce access to medicines can become “politically unpalatable for U.S. and EC government and corporate elites” (Shaffer, 2004a: 476; Sell and Prakash, 2004: 163-65). By mobilizing to reduce domestic political support for the status quo in the most economically powerful and influential countries, “developing countries retain greater leeway to formulate intellectual property policies to fit their own needs” (Shaffer, 2004a: 481). Developing countries will need considerable support to enable them to resist pressures to adopt TRIPS-Plus provisions in bilateral and regional agreements. Highlighting the unintended and devastating consequences of particular policies can be a powerful rhetorical strategy. The HIV/AIDS pandemic underscored just how costly overly-strong patent protection can be. The anthrax/bio-terror threat in the United States led American policy makers to threaten compulsory licensing of Bayer’s Cipro to ensure adequate supplies. The access to medicines campaign capitalized on this hypocrisy and it softened the American stance at Doha. Additionally, the SARS epidemic of spring 2003 led to an expansion of the WHO’s mandate and a further empowerment of NGOs within the public health context. In May 2003, the WHO assembly approved changes to international health regulations to strengthen the WHO’s ability “to respond to global public health threats based on information from non-government sources.”28 The looming avian flu situation has also prompted intensive discussion about access to Roche’s Tamiflu and alternative ways of making it widely available in the event of a catastrophic worldwide pandemic. 28. Posting of Mike Palmedo, mpalmedo@cptech.org, to IP-Health Listserv, Frances Williams, WHO to Gain Advisory Role on Pharmaceutical Patents, FIN. TIMES, May 28, 2003, available at http://lists.essential.org/pipermail/ip-health/2003-May/004803.html. 60 Developing countries should do what they can to preserve their autonomy in adopting intellectual property policies that suit their levels of development. They should resist TRIPS-Plus initiatives in bilateral and regional trade and investment agreements, insisting upon TRIPS as their maximum standard. They should seek out technical assistance that encourages them to use existing TRIPS flexibilities. They also need to participate in global standard-setting exercises concerning competition policy and address the way that they would like to regulate foreign firms' acquisition of local firms (Barton 2003: 14). Promoting genuine competition is an important policy objective. At the national level, developing countries should pursue vigorous competition legislation. Competition law can provide an important check on abuses of intellectual property rights. Even though the Reagan administration gutted American antitrust practice with respect to intellectual property rights in the name of competitiveness, consumer groups and developing countries can lobby for competition policies that check the abuses of the new “global knowledge cartels” (Drahos and Braithwaite, 2002: 206). Indeed, for most of the twentieth century American antitrust laws kept patent power in check (Sell, 2003: 5-6). Competition policies can facilitate healthy markets and keep costs down. Technical assistance directed to this end would be invaluable. In order to reduce some of the power asymmetries, it would be helpful to institutionalize expertise and technical support for developing country delegations in Geneva. Drahos has suggested the establishment of a “counter Quad” for developing countries that would function somewhat like the Cairns Group of agricultural exporters (2003: 96). The idea would be to provide continuity and technical support on diverse issues to help balance information and expertise asymmetries between the resourceabundant delegations and most of those from developing countries. This expertise would need to be provided by developing countries’ representatives and experts eager to protect developing countries’ interests. Support provided by the Quaker United Nations Offices in Geneva during the negotiations over the Doha Declaration on TRIPS and Public Health is a good example of this latter type of assistance. UNCTAD, in conjunction with ICSTD, also has assembled a panel of intellectual property experts that have helped craft reports and documents to assist developing countries’ negotiators in negotiating intellectual property issues. Developing countries could use their own version of the industry-led IPC and ABIA to counteract foreign pressure. Developing countries also could exploit the domestic critiques of the American patent system, as documented by Jaffe and Lerner (2004). In large measure the U.S. is seeking to universalize its own patent system. To the extent that the existing system is pathologically flawed, as critics contend, negotiators can challenge the adoption of the system elsewhere. At WIPO’s Open Forum on the Draft Substantive Patent Law Treaty (SPLT) in March 2006, a number of commentators pointed out pervasive problems with the U.S. system. For example, Duke Law Professor Jerome Reichman pointed out that ”no one in the developed world at the moment really knows what a proper functioning patent system for the twenty-first century should look like. . . . [T]he United States’ patent system is in a dreadful mess and badly needs reform” (2006: 6). Given this state of affairs it makes no sense to try to impose this “dysfunctional” system on the rest of the world (Reichman, 2006: 14). Argentine economist Carlos Correa warned that developing countries would be 61 harmed if induced to import a patent regime increasingly seen as “malfunctioning in developed countries, and often stifling rather than promoting innovation” (Correa, 2005b: 7). This critical perspective is not limited to a handful of professors; indeed, the U.S. Federal Trade Commission and the National Academies have both issued highly critical reports of the current system (Federal Trade Commission, 2003; National Research Council, 2004). Developing countries should also exploit differences between the U.S. and the EU over such issues as software patents, business method patents (neither of which the EU allows; Reichman, 2006), as well as their different positions on geographical indications and disclosure. Developing countries may be able to solicit EU support for a mandatory disclosure requirement in exchange for supporting the EU’s geographical indications proposals. Developing countries may be able to exploit US and EU differences over price controls for pharmaceuticals (Abbott, 2005). Furthermore, in the FTA context, developing country negotiators may be able to identify American industry allies that also object to high pharmaceutical prices. Large employers like General Motors have to pay increasingly high rates for medical plans for their employees and escalating prescription drug prices are a substantial component of the burden. Developing countries need to maintain coalition solidarity and resist US efforts to “divide and rule” (Odell, 2006). As far as access to educational materials is concerned, Ruth Okediji advocates an international principle of fair use, or fair dealing, to maintain limitations on copyright. Restrictions on copyright for educational purposes and for the dissemination of scientific information are important components of economic development and innovation. Reproduction and translation rights “operate in tandem as barriers to access in developing countries” (Okediji, 2005: 24). Limits and exceptions to copyrights are “important strategic and doctrinal tools to facilitate economic development by providing citizens with the basic means to engage in intellectual endeavors and to participate in the global knowledge economy” (Okediji, 2005: 40). For copyright, CPTech has recommended the formation of collective management organizations in order to facilitate access to copyrighted works (CPTech, 2002). Open source options such as Linux and Creative Commons licenses are important tools to expand access. Many commentators have advocated private-public partnerships to develop and disseminate medicines, copyrighted works and crop technologies. An example of a global private-public partnership is the Global Alliance for Vaccines and Immunizations (GAVI) launched by Bill Gates, together with the executive heads of WHO, UNICEF, the World Bank and Merck & Co (Buse, 2002: 51). Critics of these arrangements argue that they give private sector actors special access to U.N. decision making without corresponding access for recipient countries and marginalized groups (Buse, 2002: 60). In health the privatepublic partnerships often are designed to discourage the use of compulsory licensing, stressing instead long-term drug donation programs or programs such as the Bristol-Myers Squibb and UNAIDS’ “Bridging the Gap” in southern Africa (Buse, 2002: 35). The Accelerating Access Initiative, a joint effort between U.N. agencies and five major pharmaceutical companies, to provide discounted AIDS drugs was launched after intense public outcry and in an effort to head off debates over differential pricing and compulsory licensing (Kapczynski et al, 2005). Critics worry that these arrangements may create long- 62 term dependence and/or vulnerability. public goods. In any case, they are no panacea for providing Ever since the passage of the Bayh-Dole Act of 1980, when American universities could patent the fruits of federally funded research, they have had incentives to assert proprietary rights over their innovations. Universities play an important role in innovation. They may feel caught between the conflicting imperatives of attracting private sector funding and generating revenue through patenting activity on the one hand, and promoting public goods through “humanitarian IP” policies on the other. The choices may not be so stark, and there may be ways to navigate the contours of the current system to better balance competing imperatives. Universities could play a significant role in preserving the balance between exclusion and access. Land grant universities must continue to make available the fruits of their research to those who need it most on terms that the recipients can live with (Schuh, 2004: 359-371). For example, in the medicines sector, there could be clauses in agreements to allow a university to sub-license to generic manufacturers if its patent conflicts with efforts to distribute affordable drugs for HIV/AIDS victims in subSaharan Africa. Rai and Eisenberg offer a modest and sensible suggestion that for publicly-funded research “decisions about the dividing line between the public domain and private property should be made by institutions that are in a position to appreciate the tensions between widespread access and preservation of commercial incentives without being unduly swayed by institutional interests that diverge from the overall public interest” (2003: 303). In other words, they argue that public funding agencies should decide what fruits of their investments to patent. They also advocate addressing the upstream /downstream research tool issue by devising “a system that distinguishes cases in which proprietary claims make sense from cases in which they do not” (Rai and Eisenberg, 2003: 303). Research tool exemptions would be useful to help to preserve the domain of open science. In biotechnology a number of projects and experiments are currently underway and are modeled after the open source software movement. Stephen Maurer, Arti Rai and Andrej Sali have advocated an open source drug discovery approach to reduce costs of drugs designed for tropical diseases (Maurer, et. al: 2004). A number of universities have granted the nonprofit drug company OneWorld Health exclusive licenses to compounds and medicines (Kapczynski et al: 2005). Amy Kapczynski, Samantha Chaifetz, Zachary Katz, and Yochai Benkler have worked on a model Equitable Access License at Yale University under the auspices of Universities Allied for Essential Medicines (Kapczynski et. al: 2005). An Australian initiative, Biological Innovation for Open Society (BIOS) 29 has adapted the free software movement to agricultural biotechnology to catalyze a new selfsustaining commons for researchers. BIOS aims to create portfolios of essential research tools and to license them under a license modeled after the GNU General Public License (GPL) (Kapczynski et al: 2005). Richard Jefferson, scientist and initiator of BIOS, stated that, “’so much of what we want to do is all tied up in somebody’s intellectual property. . . . It’s a complete sclerotic mess, where nobody has any freedom of movement. Everything that open source has been fighting in software is exactly where we find ourselves now with 29 http://www.bios.net 63 biotechnology’” (quoted in Kapczynski et al: 2005). Jefferson has created two technologies that circumvent proprietary tools for biotechnological crop improvement. One of these technologies, a method for introducing new genes into plants, bypasses numerous patents and has been successfully used in a Chinese project to create transgenic rice lines (Feldman, 2004: 127). The Public Intellectual Property Resource for Agriculture (PIPRA) seeks to facilitate the development and dissemination of crops for developing countries and promote commons-based crop development.30 In addition to these efforts to try to reclaim the commons and facilitate access, some have promoted the concept of awarding prizes for innovation and developing R&D treaties to require contributions toward the development of neglected public goods (Hubbard and Love, 2004). One very broad-based movement is the support for an “access to knowledge” treaty that would begin to construct an alternative framework for intellectual property.31 This treaty initiative came out of WIPO’s decision to examine proposals for a development agenda in 2004 (Drahos, 2005: 15). As Drahos suggest, “the crucial conceptual move . . . would be for the treaty to link the instrumental status of intellectual property to the promotion of . . . basic rights” such as the rights to food, health, and education (Drahos, 2005: 17). In other words it would be targeted at restoring intellectual property’s status as a servant of broader goals rather than the master (as it seems to be today). Casting the treaty effort as a human rights approach might work insofar as it is a familiar norm that states already have publicly committed to. As Rosemary Coombe points out, “social networks are becoming more vocal . . . and link together hitherto unimagined coalitions of environmentalists, feminists, farmers, food and health activists, indigenous peoples, and religious groups in the articulation of alternative moral economies of value” (Coombe, 2004). Cross-sectoral mobilization and the broadening of participation under a common frame are necessary if champions of an alternative are to prevail. While the different access campaigns have different particular concerns, they could unite under an access to knowledge banner. 30 http://www.pipra.org 31 http://www.cptech.org/a2k/ 64 References Abbott, Frederick, 2000. “Distributed Governance at the WTO-WIPO: An Evolving Model for Open-Architecture Integrated Governance” Journal of International Economic Law 3: 63 Abbott, Frederick, 2002a. “The Doha Declaration on the TRIPS Agreement and Public Health: Lighting a Dark Corner at the WTO” Journal of International Economic Law 5: Abbott, Frederick, 2002b. “Compulsory Licensing for Public Health Needs: the TRIPS Agenda at the WTO after the Doha Declaration on Public Health,” Occasional Paper 9. February. Geneva: Quaker United Nations Office. http://www.afsc.org/quno.htm Abbott, Frederick, 2004. “The Doha Declaration on the TRIPS Agreement and Public Health and the Contradictory Trend in Bilateral and Regional Free Trade Agreements” Quaker United Nations Office, Occasional Paper 14 (April). http://www.quno.org Abbott, Frederick, 2005. “The WTO Medicines Decision: World Pharmaceutical Trade and the Protection of Public Health” American Journal of International Law 99: 317-358. ActionAid International, 2006. “Under the Influence: Exposing Undue Corporate Influence over Policy-Making at the World Trade Organization” at: http://www.actionaid.org Albin, Cecilia, 2001. Justice and Fairness in International Negotiation Cambridge: Cambridge University Press. Alvarez, Jose, 2001. “Trade and the Environment: Implications for Global Governance: How Not to Link: Institutional Conundrums of an Expanded Trade Regime” Widener Law Symposium Journal 7: 1 Alvarez, Jose, 2002a. “The WTO as Linkage Machine” American Journal of International Law 96: 146 Alvarez, Jose, 2002b. “Foreword: the Boundaries of the WTO” American Journal of International Law 96: 1 American BioIndustry Alliance, 2005: “Views on the Establishment of an ABS/IR” (October 12). http://www.abialliance.com Aoki, Keith 2003. "Weeds, Seeds & Deeds: Recent Skirmishes in the Seed Wars", 11 Cardozo Journal of International and Comparative Law (Summer): 247. Arup, Christopher, 1998. “Competition Over Competition Policy for international Trade and Intellectual Property” Prometheus, 16: 367-81. 65 Attaran, Amir and Lee Gillespie-White, 2001. “Do Patents for Antiretroviral Drugs Constrain Access to AIDS Treatment in Africa? Journal of the American Medical Association 286: 1886-1892. Baker, Andrew, 2000. "Globalization and the British 'Residual' State" in Richard Stubbs and Geoffrey Underhill eds. Political Economy and the Changing Global Order 2nd ed. (Oxford: Oxford University Press): 362-372. Barton, John 2003. “Nutrition and Technology Transfer Policies”, UNTAD/ICTSD Capacity Building project on Intellectual Property and Sustainable Development” (August). http://www.iprsonline Basu, Sanjay, 2003. “Patents and Pharmaceutical Access” May 29. http://www.zmag.org/content/showarticle.cfm?ItemID=3694 Bate, Roger and Richard Tren, 2003. “Do NGOs Improve Wealth and Health in Africa? http://www.aei.org/docLib/20030612_batepub.pdf Benvenisti, Eyal and George Downs, 2004. “Distributive Politics and International Institutions: the Case of Drugs” Case Western Reserve Journal of International Law 36: 21-52. Bhagwati, Jagdish, 2002. “Afterword: The Boundaries of the WTO: the Question of Linkage” American Journal of International Law 96: 126 Bloche, Greg, 2002. “WTO Deference to National Health Policy: Toward an Interpretive Principle” Journal of International Economic Law 5: 825 Bond, Patrick, 1999. “Globalization, Pharmaceutical Pricing, and South African Health Policy: Managing Confrontation with U.S. Firms and Politicians” International Journal of Health Services 29, 4: 765-792. Boyle, James, 1992."A Theory of Law and Information: Copyright, Spleens, Blackmail, and Insider Trading", California Law Review 80: 1415-1540. Braithwaite, John and Peter Drahos, 2000. Global Business Regulation Cambridge: Cambridge University Press. Busaniche, Beatriz, 2005. Interview with Beatriz Busaniche, Seedling October. http://www.grain.org/convergence/?id=103 Buse, Kent et. al. 2002. “Globalisation and Health Policy: Trends and Opportunities” in Kelly Lee et al eds., Health Policy in a Globalising World Cambridge: Cambridge University Press Calfee, John, 2003. “Patently Wrong: Free Drugs are No Panacea for Poor Nations” 66 Washington Times January 28: A21. Carrier, Michael 2003. "Resolving the Patent-Antitrust Paradox through Tripartite Innovation", 56 Vanderbilt Law Review 1047. Case, Mary M. 2005. “Increasing Concentration in the Publishing Industry: A Recent History of Publisher Mergers and pricing in the Literature of Science, Technology, Medicine and Law” (power point presentation on file with author). Cerny, Philip, 2000. "Political Globalization and the Competition State", in Richard Stubbs and Geoffrey Underhill eds, Political Economy and the Changing Global Order 2nd ed. (Oxford: Oxford University Press): 300-309. Chapman, Audrey, 2002. “The Human Rights Implications of Intellectual Property Protection” Journal of International Economic Law 5: 861 Charnovitz, Steve, 2002. “The Legal Status of the Doha Declaration” Journal of International Economic Law 5: 207 Checkbiotech.org, 2006. “Biotech Firms form alliance TRIPS” January 10. http://www.checkbiotech.org Coleman, William and Melissa Gabler, 2002. “Agricultural Biotechnology and Regime Formation: a Constructivist Assessment of the Prospects” International Studies Quarterly 46: 481-506. Commission on Intellectual Property Rights, 2002. Integrating Intellectual Property Rights and Development Policy http://www.iprcommission.org/papers/text/final_report/reportwebfinal.htm. Comor, Edward. 2001. “The Role of Communications in Global Civil Society: Forces, Processes, Prospects” International Studies Quarterly 45 Consumer Project on Technology, 2005. “Statement of CPTech on TRIPS Amendment” 6 December 2005, http://www.cptech.org/ip/wto/p6/cptech12062005.html Consumer Project on Technology, 2005. “WTO Members Should Reject Bad Deal on Medicines: Joint Statement by NGOs on TRIPS and Public Health” 3 December. http://www.cptech.org/ip/wto/p6/ngos12032005.html Consumer Project on Technology, 2002 “CPTech’s Page on Collective Management of Copyrights” http://www.cptech.org/cm/copyrights.html Consumer Project on Technology, 2000. “Background Information on fourteen FDA Approved HIV/AIDS Drugs” June 8. http://www.cptech.org/ip/health/aids/druginfo.html 67 Consumer Project on Technology et al., 2001. “Comment on the Attaran/Gillespie-White and PhRMA Surveys of Patents on Antiretroviral Drugs in Africa, October 16. http://www.cptech.org/ip/health/africa/dopatentsmatterinafrica.html Coombe, Rosemary, 1998. “Intellectual Property, Human Rights and Sovereignty: New Dilemmas in International Law Posed by the Recognition of Indigenous Knowledge and the Conservation of Biodiversity” Indiana Journal of Global Legal Studies 6. Coombe, Rosemary, 2004. “Commodity Culture, Private Censorship, Branded Environments, and Global Trade Politics: Intellectual Property as a Topic of Law and Society Research” Austin Sarat, ed. The Blackwell Companion to Law and Society Malden, MA.: Basil Blackwell: 369-391. Correa, Carlos, 2002. “TRIPS and Access to Medicines: Internationalization of the Patent System and New Technologies” Wisconsin International Law Journal 20: 523 Correa, Carlos, 2004. “Investment Protection in Bilateral and Free Trade Agreements: Implications for the Granting of Compulsory Licenses” Michigan Journal of International Law 26: 331-53. Correa, Carlos, 2005a. “The Politics and Practicalities of a Disclosure of Origin Obligation”, Quaker United Nations Office, Occasional Paper 16 (January). http://www.geneva.quno.org Correa, Carlos, 2005b. “An Agenda for Patent Reform and Harmonization for Developing Countries”, prepared for the Bellagio Dialogue on “Intellectual Property and Sustainable Development: Revising the agenda in a new context”, International Center for Trade and Sustainable Development ) ICTSD) 24-28 September. Bellagio, Italy. On file with author. Crawford, Neta, 2002. Argument and Change in World Politics: Ethics, Decolonization and Humanitarian Intervention, Cambridge: Cambridge University Press. Doern, Bruce, 1999. Global Change and Intellectual Property Agencies N.Y.: Routledge Doctors Without Borders, 2005. “The Second Wave of the Access Crisis: Unaffordable AIDS Drug Prices Again” December 10. available at: http://www.doctorswithoutborders.org/news/hiv-aids/briefing_doc_12-10-2005. Drahos, Peter, 2001. “BITS and BIPS: Bilateralism in Intellectual Property” Journal of World Intellectual Property Law 4, 6 (November). Drahos, Peter, 2002. “Developing Countries and Intellectual Property Standard-Setting” Journal of World Intellectual Property 5, no. 5: 765-789. Drahos, Peter and John Braithwaite, 2002. Information Feudalism, London: Earthscan Publications, Ltd. 68 Peter Drahos, 2003. “When the Weak Bargain with the Strong: Negotiations in the World Trade Organization” International Negotiation 8: 79Drahos, Peter. 2004. “Securing the Future of Intellectual Property: Intellectual Property Owners and their Nodally Coordinated Enforcement Pyramid” Case Western Reserve Journal of International Law 36:1: 53-78. Drahos, Peter, 2005. “An Alternative Framework for the Global Regulation of Intellectual Property Rights” Centre for Governance of Knowledge and Development, Working Paper. Regulatory Institutions Network, the Australian National University. http://cgkd.anu.edu.au/menus/workingpapers.php Dunoff, Jeffrey, 2001. “”The WTO in Transition: Of Constituents, Competence and Coherence” George Washington International Law Review 33: 979 Dutfield, Graham. 2005a. “The US and Europe are ‘Intellectual Property Fundamentalists”, SciDev.Net, March 11. http://www.scidev.net/dossiers Dutfield, Graham. 2005b. “Thinking Aloud on Disclosure of Origin” Quaker United Nations Office, Occasional Paper 18 (October). Dutfield, Graham, 2003a. Intellectual Property Rights and the Life Science Industries (Aldershot, UK: Ashgate Publishing Limited). Dutfield, Graham. 2003b. "Sharing the Benefits of Biodiversity: Is There a Role for the Patent System?" Journal of World Intellectual Property Dutfield, Graham, 2003c. “Should We Terminate Terminator Technology?” European Intellectual Property Review, 491-495, Dutfield, Graham, 2002. “Trade, Intellectual Property and Biogenetic Resources: A Guide to the International Regulatory Landscape” http://www.quno.org Eisner, Marc, 1991. Antitrust and the Triumph of Economics: Institutions, Expertise, and Policy Change, Chapel Hill, University of North Carolina Press. Federal Trade Commission, 2003. “To Promote Innovation: The Proper Balance of Competition and Patent Law and Policy” http://www.ftc.gov/os/2003/10/innovationrpt.pdf Feldman, Robin, 2004. “The Open Source Biotechnology Movement: Is It Patent Misuse?” Minnesota Journal of Law, Science & Technology 6:1: 117-167. Fligstein, Neil, 1996. "Markets as Politics: A Political-Cultural Approach to Market Institutions", American Sociological Review 61 (August): 656-673. 69 Food and Agriculture Organization (FAO), 2004. “Voluntary Guidelines to Support the Progressive Realization of the Right to Adequate Food in the Context of National Food Security. http://www.fao.org/righttofood Foer, Albert, 2005. “Antitrust Perspectives on Mergers in the Academic Publishing Industry” presented at The Information Access Alliance and the American Antitrust Institute Invitational Symposium on Antitrust Issues in Scholarly and Legal Publishing” Washington, D.C. February 11. (on file with author). Fowler, Cary, 1994. Unnatural Selection: Technology, Politics and Plant Evolution Yverdon, Switzerland: Gordon and Breach. GAIA/GRAIN, 1998a. "Intellectual Property Rights and Biodiversity: the Economic Myths" Global Trade and Biodiversity in Conflict 3: 1-20, http://www.grain.org/publications/gtbc/issue3.htm GAIA/GRAIN, 1998b. "TRIPS versus CBD", Global Trade and Biodiversity in Conflict, Issue No. 1 (April). http:www.grain.org/publications/issue1-en-p.htm GAIA/GRAIN, 1999. "UPOV on the War Path", Seedling (June). http://www.grain.org/publications/jun99/jun991.htm Gathii, James, 2001. “Institutional Concerns of an Expanded Trade Regime: Where Should Global Social and Regulatory Policy Be Made?: Re-characterizing the Social in the Constitutionalization of the WTO: A Preliminary Analysis” Widener Law Symposium Journal 7: 137 Gathii, James, 2002. “The Legal Status of the Doha Declaration on TRIPS and Public Health Under the Vienna Convention on the Law of Treaties” Harvard Journal of Law & Technology 15: 291 Gellman, Barton, 2000. “Gore in Conflict for Health and Profit” The Washington Post May 21. http://www.washingtonpost.c...rticle&nodecontentID=A41297-2000May20. Germain, Randall, 2000. "Globalization in Historical Perspective" in R. Germain, ed. Globalization and Its Critics London: Macmillan: 67-90. Hammer, Peter 2002. “Differential Pricing of Essential AIDS Drugs: Markets, Politics and Public Health”, Journal of International Economic Law 5:4 (December): 883-912. Gorlin, Jacques, 2006. “Additional Patent Disclosure Requirements for Biotech Inventions in Play in WTO, WIPO and CBD” Stockholm Network: Know IP – Stockholm Network Monthly Bulletin on IPRs (February) 2:1: 3-4. Grabowski, Henry. 2002. “Patents, Innovation and Access to New Pharmaceuticals” Journal of International Economic Law 5: 849 70 GRAIN, 2005. “The FAO Seed Treaty: From Farmers’ Rights to Breeders’ Privileges”, Seedling (October) http://www.grain.org/seedling/?id=411 Hayslett III, T. 1996. "1995 Antitrust Guidelines for the Licensing of Intellectual Property: Harmonizing the Commercial Use of Legal Monopolies with the Prohibitions of Antitrust Law" Journal of Intellectual Property Law 3 (Spring): 375-405. Heald, Paul, 2003. "The Rhetoric of Biopiracy", 11 Cardozo Journal of International and Comparative Law (Summer): 519. Helfer, Laurence, 2002. “Intellectual Property Rights in Plant Varieties: an Overview with options for National Governments” FAO Legal Papers Online. http://www.fao.org/Legal/pub-e.htm Helfer, Laurence, 2004. “Regime Shifting: The TRIPs Agreement and New Dynamics of International Intellectual Property Lawmaking” Yale Journal of International Law 1, 71 Hepburn, Jonathan 2002. “Negotiating Intellectual Property: Mandates and Options in the Doha Work Program,” Occasional Paper 10. November. Geneva: Quaker United Nations Office. http://www.quno.org ‘t Hoen, Ellen, 2002. “TRIPS, Pharmaceutical Patents and Access to Essential Medicines: A Long Way from Seattle to Doha” Chicago Journal of International Law 3: 27 Hoff, Paul, 1986. Inventions in the Marketplace: Patent Licensing and the U.S. Antitrust Laws Washington, D.C. American Enterprise Institute. Howse, Robert, 2001. “The Boundaries of the WTO: From Politics to Technocracy – and Back Again: The Fate of the Multilateral Trading Regime” American Journal of International Law 96: 94 Howse, Robert and Makau Mutua, 2000. “Protecting Human Rights in a Global Economy: Challenges for the World Trade Organization” International Centre for Human Rights and Democratic Development, http://www.ichrdd.ca/english/commdoc/publications/globalization/wtoRightsGlob.html Hubbard, Tim and James Love, 2004. “A New Trade Framework for Global Healthcare R&D” http://biology.plos.journals.org/archive/15457885/2/2/pdf/10.1371_journal.pbio.0020052-S.pdf Institute for Agriculture and Trade Policy, 2005. “Planting the Rights Seed: A Human Rights Perspective on Agriculture and Trade in the WTO”, Backgrounder No. 1 in the Thread series. http://www.iatp.org and http://www.3dthree.org 71 International Centre for Trade and Sustainable Development, 2004. “Brazil Wins Landmark Cotton Dispute” Bridges (May): 7. International Centre for Trade and Sustainable Development, 2005. “WIPO Development Agenda Status Unclear” Bridges (September/October): No. 9: 22. IP Blog, 2005. “WTO Extends Deadline for Poor Countries to Implement TRIPS by Seven Years” 29 November. http://www.ipblog.org/blog/IPBlog.nsf/dx/wto-extendsdeadline-for-poor-countries-to-imp... Ip-health, 2001. “Re: Call for Endorsements on Glivec from South Korea” November 30. http://lists.essential.org/pipermail/ip-health/2001-November Ip-health, 2003. “Text of Korean Decision in Glivec Case” March 10. http://www.lists.essential.org/pipermail/ip-health/2003/March Ip-health, 2005. “Deadlock on TRIPS and Public Health Deliberations on a “Permanent Solution” October 28, 2005. http://lists.essential.org/pipermail/ip-health/2005-October/008531.html Ip-health digest, 2006; Vol.1 #1875, Feb. 24. http://lists.essential.org/pipermail/iphealth/2006-February IP-Watch, 2005. “Nations Clash on Future of WIPO Development Agenda” 11 April. http://www.ip-watch.org IP-Watch, 2005. “Non-Profits, Industry Offer Views on WIPO Development Agenda” 14 April. http://www.ip-watch.org IP-Watch, 2005. “Avian Flu Issues Could Arise at TRIPS Council Meeting,” 25 October. http://www.ip-watch.org IP-Watch, 2005. “IP Issues Reappear on Agenda of WTO Talks” 14 October. http://www.ip-watch.org IP-Watch, 2005. “Official: In WTO Talks, US Pushes Russia to Restrictive TRIPS Standard” 24 October. http://www.ip-watch.org IP-Watch, 2005. “Pandemic Fears Raise Questions about WTO Health Waiver Opt-Out” 27 October. http://www.ip-watch.org IP-Watch, 2005. “TRIPS Council Issues Still Alive for WTO Ministerial” 28 October. http://www.ip-watch.org IP-Watch, 2005. “EU Elevates Geographical Indications as Priority for Hong Kong” 28 October. http://www.ip-watch.org 72 IP-Watch, 2005. “Interview with Eric Smith, President, International Intellectual Property Alliance” 4 November. http://www.ip-watch.org IP-Watch, 2005. “Industry Concerned about Development Agenda at WTO” 4 November. http://www.ip-watch.org IP-Watch, 2005. “African Countries Ready to Accept TRIPS and Public Health Deal” 6 December, 12:36am. http://www.ip-watch.org IP-Watch, 2005. “WTO Strikes Agreement on TRIPS and Public Health on Eve of Ministerial” 6 December, 10:53pm. http://www.ip-watch.org IP-Watch, 2005. “TRIPS Health Amendment Evokes Harsh NGO Reaction, Industry Caution” 7 December. http://www.ip-watch.org International Treaty on Plant Genetic Resources for Food and Agriculture http://www.fao.org/ag/cgrfa/itpgr.htm IP-Watch, 2005. “Experts Debate IP Issues as Hong Kong WTO Ministerial Opens” 13 December. http://www.ip-watch.org IP-Watch, 2005. “TRIPS Public Health Announcement Questioned ; China Implements Decision” 14 December. http://www.ip-watch.org IP_Watch, 2005. “India, Brazil Tie Biodiversity Negotiations to Doha Development Package” 15 December. http://www.ip-watch.org IP-Watch, 2005. “Peru Attempts Strong WTO Position on Disclosure Despite Weaker US Deal” 17 December). http://www.ip-watch.org IP-Watch, 2005. “Latest WTO Ministerial Draft Urges Discussion on IP Issues to Intensify, Adds Review Date” 18 December, 11:51am. http://www.ip-watch.org IP-Watch, 2006. “Biotech Firms Form Alliance to Fight Off Possible TRIPS Amendments” 6 January. http://www.ip-watch.org IP-Watch, 2006a. “The Hunt is on to Advance or End Development Agenda at WIPO”, 24, February. http://www.ip-watch.org IP-Watch, 2006b. “Industry’s Take on WIPO Development Talks: An Interview with Director General of CropLife”, 24 February. http://www.ip-watch.org IP-Watch, 2006c. “Agricultural IP Issues Debated at Industry Side Event to WIPO Meeting” 24 February. http://www.ip-watch.org 73 IP-Watch, 2006d. “Biotech Industry Fights Disclosure in Patents on Three IP Policy Fronts” 2 March. http://www.ip-watch.org Ip-Watch, 2006e. “Groups Decry Impact of IP and Health Terms in US Trade Agreements” 3 March. http://www.ip-watch.org International Chamber of Commerce, 2005. “Current and Emerging Intellectual Property Issues for Business: A Roadmap for Business and Policymakers” http://www.iccwbo.org Irwin, Douglas, 2002. Free Trade under Fire Princeton: Princeton University Press. Jackson, John, 2002. “Afterword: The Linkage Problem – Comment on Five Texts” American Journal of International Law 96: 118Jackson, Patrick, 2002. “Jeremy Bentham, Foreign Secretary: or, the Opportunity Costs of Neo-Utilitarian Analyses of Foreign Policy” Review of International Political Economy, 9: Jaffe, Adam and Josh Lerner, 2004. Innovation and Its Discontents: How our Broken Patent System is Endangering Innovation and Progress, and What to Do about it Princeton: Princeton University Press. Jones, Bryan. 2001. Politics and the Architecture of Choice: Bounded Rationality and Governance Chicago: University of Chicago Press. Kapczynski, Amy, Samantha Chaifetz, Zachary Katz, and Yochai Benkler, 2005. “Addressing Global Health Inequities: An Open Licensing Approach for University Innovations” The Berkeley Technology Law Journal Kuanpoth, Jakkrit, 2006. “Closing in on Biopiracy: Legal Dilemmas and Opportunities for the South” in Ricardo Melendez-Ortiz and Vicente Sanchez, Trading in Genes: Development Perspectives on Biotechnology, Trade and Sustainability London: Earthscan: 139-52. Keck, Margaret and Kathryn Sikkink, 1998. Activists without Borders: Advocacy Networks in International Politics Ithaca: Cornell University Press. Kloppenburg, Jack, 2005. “Interview with GRAIN” Seedling (October) http://www.grain.org/seedling/?id=414 Lamptey, Peter, 2003. “Future Challenges in the Global Fight against HIV/AIDS in Developing Countries” Emory International Law Review 17:2: 645-661. Lettington, Robert 2003. "Small-scale Agriculture and the Nutritional Safeguard under Article 8(1) of the Uruguay Round Agreement on Trade-Related Aspects of Intellectual Property Rights: Case Studies From Kenya and Peru" (November) get URL from Bridges 74 Lieberwitz, Risa, 2004. "Book Review: The Marketing of Higher Education: The Price of the University's Soul: Universities in the Marketplace: The Commercialization of Higher Education. By Derek Bok." 89 Cornell Law Review (March): 763. Love, James, 2005. “No Gift to the Poor: Strategies used by US and EC to protect big pharma in WTO TRIPS negotiations”, December 1st, Working Agenda available at: http://workingagenda.blogspot.com/2005/12/no-gift-to-poor-strategies-used-by-us.html Marden, Emily, 1999. “The Neem Tree Patent: International Conflict over the Commodification of Life” Boston College Environmental Affairs Law Review 22: 279-295. Maskus, Keith and Jerome Reichman, 2004. “The Globalization of Private Knowledge Goods and the Privatization of Global Public Goods” Journal of International Economic Law 279. Matthews, Duncan, 2004a. “WTO Decision on Implementation of Paragraph 6 of the Doha Declaration on the TRIPS Agreement and Public Health: A Solution to the Access to Essential Medicines Problem?” Journal of International Economic Law 7 (1): 73-93. Matthews, Duncan, 2004b. “Is History Repeating Itself? Outcome of the Negotiations on Access to Medicines, the HIV/AIDS Pandemic and Intellectual Property Rights in the World Trade Organisation” Electronic Law Journal, LGD 2004(1) http://www2.warwick.ac.uk/fac/soc/law/elj/lgd/2004_1/matthews/ Maurer, Stephen, Arti Rai and Andrej Sali, 2004. “Finding Cures for Tropical Diseases: Is Open Source and Answer?” http://salilab.org/pdf/136_MaurerBIOESSYS2004.pdf May, Christopher and Susan K. Sell, 2006. Intellectual Property Rights: A Critical History Boulder: Lynne Rienner Publishers. McDowell, Natasha, 2003. “WTO Patent Rules Should be Scrapped” Science and Development Network, http://www.scidev.net/NEWS/index.cfm?fuseaction=readnews&itemid=429&language=1 (February 26th). Medecins sans Frontieres, Health Action International, and Consumer Project on Technology, 1996. “Amsterdam Statement” November 25-26. http://www.cptech.org/ip/health/amsterdamstatement/html Merges, Robert. 2000. “One Hundred Years of Solicitude: Intellectual Property Law, 1900-2000. California Law Review 88: 2187-2240. Muller, Harald, 2001. “International Relations as Communicative Action” in Karin Fierke and Knud Jorgensen eds. Constructing International Relations: The Next Generation N.Y.: M.E. Sharpe 75 Munroe, Mary H. 2005. “The Academic Publishing Industry: A Story of Mergers and Acquisitions” http://www.niulib.niu.edu/publishers/ (Sept. 30 update). (visited 2/23/06). Musungu, Sisule, 2005. “Rethinking Innovation, Development and Intellectual Property in the UN: WIPO and Beyond” TRIPS Issues Papers 5, Quaker International Affairs Programme, Ottawa Canada. Nachtigal, Nicole, 2001. “Casenote: A Modern David and Goliath Farmer v. Monsanto: Advising a Grower on the Monsanto Technology Agreement 2001” Great Plains Natural Resources Journal 6: 50-76. National Research Council, 2004. A Patent System for the 21st Century Washington, D.C.: National Academy Press Newell, Peter and Dominic Glover, 2003. "Globalisation and the International Governance of Modern Biotechnology. Business and Biotechnology: Regulation and the Politics of Influence" Institute of Development Studies. http://www.gapresearch.org Newfarmer, Richard et al., 2001. “Global Economic Prospects and the Developing Countries” http://www.worldbank.org/prospects/gep2002/gep2002complete.pdf Odell, John, 2006. Negotiating Trade: Developing Countries in the WTO and NAFTA Cambridge: Cambridge University Press. Odell, John and Susan K. Sell, 2006. “Reframing the Issue: the WTO Coalition on Intellectual Property and Public Health, 2001” in Odell, ed. Negotiating Trade: Developing Countries in the WTO and NAFTA Cambridge: Cambridge University Press: 85-114. Okediji, Ruth, 2005. “The International Copyright System: Limitations, Exceptions and Public Interest Considerations for Developing Countries in the Digital Environment” International Centre for Trade and Sustainable Development, http://www.iprsonline.org Omer, Assad. 2000. “An Overview of Legislative Changes” in Surendra Patel et al. eds, International Technology Transfer: the Origins and Aftermath of the United Nations Negotiations on a Draft Code of Conduct Springer. 295 Patent and Trademark Office, 2005. “Patent Law Harmonization Talks Stall; Brazil, Argentina, India Oppose Compromise” June 14. http://www.uspto.gov/main/homepagenews/bak2005jun14.htm Pauwelyn, Joost, 2001. “The Role of Public International Law in the WTO: How Far Can We Go?” American Journal of International Law 95: 535 Phillips, Ronald, 2004. “Intellectual Property Rights for the Public Good: Obligations of U.S. Universities to Developing Countries” Minnesota Journal of Law, Science & Technology 6:1 177-185. 76 Pistorius, Robin and Jeroen van Wijk, 1999. The Exploitation of Plant Genetic Information: Political Strategies in Crop Development N.Y.: CABI Publishing. Rai, Arti and Rebecca Eisenberg, 2003. "The Public Domain: Bayh-Dole Reform and the Progress of Biomedicine" 66 Law & Contemporary Problems (Winter/Spring): 289. Raustiala, Kal and David Victor, 2003. “The Regime Complex for Plant Genetic Resources”, Working Paper 14, Program on Energy and Sustainable Development, Stanford University (on file with author). Reichman, Jerome, 2006. “Patent Law Harmonization and the Draft SPLT” http://www.wipo.org Reichman, Jerome and David Lange, 1998. “Bargaining around the TRIPS Agreement: The Case for Ongoing Public-Private Initiatives to Facilitate Worldwide Intellectual Property Transactions” Duke Journal of Comparative and International Law 9: 11 Reichman, Jerome and Paul Uhlir 2003. “A Contractually Reconstructed Research Commons for Scientific Data in a Highly Protectionist Intellectual Property Environment” Law and Contemporary Problems 66: 315-462. Reid, W. and S. Laird, C. Meyer, R. Gamez, A. Sittenfeld, D. Janzen, M. Gollin and C. Juma, 1993. Biodiversity Prospecting: Using Genetic Resources for Sustainable Development Washington, D.C.: World Resources Institute. Reuters, 2003. “Pfizer’s McKinnell Says Drug Patent Talks Progress” January 28. http://lists.essential.org/pipermail/ip-health/2003-January/004184.html Rosenberg, Barbara, 2006. “Market Concentration of the Transnational Pharmaceutical Industry and the Generic Industries: Trends on Mergers, Acquisitions and Other Transactions” in Pedro Roffe, Geoff Tansey and David Vivas-Eugui, Negotiating Health: Intellectual Property and Access to Medicines London: Earthscan Rosenberg, Tina, 2001. “Look at Brazil” The New York Times Jan. 28 (magazine). Schuh, G. Edward, 2004. “Intellectual Property Rights and the Land Grant Mission”, Minnesota Journal of Law, Science and Technology 6:1: 359-371. Schultz, Mark and David Walker, 2005. “How Intellectual Property Became Controversial: NGOs and the New International IP Agenda” Engage, Vol. 6:1: 82-98. http://www.ngowatch.org (visited 02/24/06) Sell, Susan K. 1998, North-South Politics of Intellectual Property and Antitrust Albany: State University of New York Press. 77 Sell, Susan K. 2002. “TRIPS and the Access to Medicines Campaign” Wisconsin International Law Journal 20: 481Sell, Susan K. 2003. Private Power, Public Law: the Globalization of Intellectual Property Rights Cambridge: Cambridge University Press. Sell, Susan K. and Aseem Prakash, 2004. “Using Ideas Strategically: The Contest between Business and NGO Networks in Intellectual Property Rights” International Studies Quarterly 48: 143-175. Shaffer, Gregory, 2001. “The World Trade Organization Under Challenge: Democracy and the Law and Politics of the WTO’s Treatment of Trade and Environment Matters” Harvard Environmental Law Review 25: 1Shaffer, Gregory. 2003. Defending Interests: Public-Private Partnerships in WTO Litigation Washington, D.C.: Brookings Institution Press. Shaffer, Gregory, 2004a. “Recognizing Public Goods in WTO Dispute Settlement: Who Participates? Who Decides?” Journal of International Economic Law 7: Shaffer, Gregory, 2004b. “Power, Global Governance and the WTO: the Need for a Comparative Institutional Approach” in Michael Barnett and Raymond Duvall eds., Power in Global Governance Cambridge: Cambridge University Press. Shiva, Vandana, 1997. Biopiracy: The Plunder of Nature and Knowledge. South End Press. Shulman, Seth. 1999. Owning the Future, New York: Houghton Mifflin Company. Smith, James McColl, “WTO and Dispute Settlement: The Politics of Procedure in Appellate Body Rulings” World Trade Review 2: 65 South Centre and Center for International Environmental Law, 2004a. “South Centre and CIEL IP Quarterly Update: Fourth Quarter, 2004” http://www.southcentre.org South Centre/CIEL (Center for International Economic Law), 2004b. “Intellectual Property and Development: Overview of Developments in Multilateral, Regional, and Bilateral Fora”, http://www.southcentre.org South Centre and Centre for International Environmental Law, 2005a. “South Centre and CIEL IP Quarterly Update: Third Quarter 2005”. http://www.southcentre/org South Centre and Center for International Environmental Law, 2005b. “South Centre and CIEL IP Quarterly Update: Second Quarter, 2005”. http://www.southcentre/org 78 Steger, Deborah, 2002. “Afterword: The Boundaries of the WTO: The ‘Trade and. . .’ Conundrum – A Commentary” American Journal of International Law 96:135Stein, Eric, 2001. “International Integration and Democracy: No Love at First Sight” American Journal of International Law 95: 489 Sutherland, Johanna 1998. “TRIPS, Cultural Politics and Law Reform” Prometheus 16:3: 291-303. Symposium, 2002. “Global Intellectual Property Rights: Boundaries of Access and Enforcement” Fordham Intellectual Property, Media and Entertainment Law Journal 12: 675 Taylor, Michael and Jerry Cayford, 2004. “Biotechnology Patents and African Food Security: Aligning America’s Patent Policies and International Development Interests”, Minnesota Journal of Law, Science and Technology (December): Vol. 6, No. 1: 277-304. Tejera, Valentina, 1999. “Tripping over Property Rights: Is it possible to reconcile the Convention on Biological Diversity with Article 27 of the TRIPS Agreement?” New England Law Review 33 (Summer): 967-987. Trebilcock, Michael and Robert Howse, 1995. The Regulation of International Trade (N.Y.: Routledge). Tversky, Amos and Daniel Kahneman, 1981. “The Framing of Decisions and the Psychology of Choice” Science, 211. UNCTAD-ICTSD, 2003, UNCTAD-ICTSD Project on IPRs and Sustainable Development http://www.iprsonline United Nations High Commissioner for Human Rights, 2001. “The Impact of the Agreement on Trade-Related Aspects of Intellectual Property Rights on Human Rights: Report of the High Commissioner” http://www.eldis.org/static/DOC5597.htm UPOV, 2002. "States Party to the International Convention for the Protection of New Varieties of Plants" http://www.upov.int/eng/ratif/pdf/ratifmem.pdf United States Trade Representative, 2006, “United States and Colombia Conclude Free Trade Agreement” February 27. http://www.ustr.gov Vaidhyanathan, Siva, 2001. Copyrights and Copywrongs: the Rise of Intellectual Property and How It Threatens Creativity NY: New York University Press. Van Wijk, Jeroen, 2004. “Terminating Piracy or Legitimate Seed Saving? The Use of Copy-Protection Technology in Seeds” Technology Analysis & Strategic Management 16: 121-141. 79 Verma, S.K. 2001. “The TRIPS Agreement and Development” in Surendra Patel et al. eds, International Technology Transfer Springer. Viana, Jose 2002. “Intellectual Property Rights, the World Trade Organization and Public Health: the Brazilian Perspective” Connecticut Journal of International Law 17: 311 Vick, Karl, 1999. “African AIDS Victims Losers of a Drug War” The Washington Post December 4: A1. Vivas-Eugui, David, 2003. “Regional and Bilateral Agreements and a TRIPS-plus World: the Free Trade Area of the Americas Agreement (FTAA)”, Quaker United Nations Office, http://www.quno.org Volansky, Mark, 2002. “Comment: Achieving Global Health: A Review of the World Health Organization’s Response” Tulsa Journal of Comparative and International Law 10: 223Washington Post, 2003. “Behind the Lobbying Curtain” June 9: A20. Wojahn, Patrick, 2001/2002. “Comment: Conflict of Rights: Intellectual Property Under TRIPS, the Right to Health, and AIDS Drugs” UCLA Journal of International Law & Foreign Affairs 6: 463World Health Organization (WHO), Essential Drugs and Medicines Home Page, http://www.who.int/medicines World Health Organization, 2000. “Revised Drug Strategy” (March 13) http://www.who.int/gb/ebwha/pdf_files/WHA53/ea10.pdf World Health Organization, 2001. “Policy Perspectives on Medicines: Globalization, TRIPS and Access to Pharmaceuticals” (March). http://www.who.int/medicines/library/edm_general/6pagers/PPM03ENG.pdf World Health Organization, 2003. “Intellectual Property Rights, Innovation and Public Health May 12. http://www.who.int/gb/ebwha/pdf_files/WHA56/ea5617.pdf World Health Organization, 2003. “Fourth Report of Committee A (Draft), May 28. http://www.who.int/gb/ebwha/pdf_files/WHA56/ea5666.pdf World Intellectual Property Organization, 2001. “Revised Draft Program and Budget 2002-2003”, http://www.wipo.int/documents/en/documents/govbody/budget/2002_03/rev/pdf/pbc4_2.p df. 80 World Trade Organization, “Declaration on the TRIPS Agreement and Public Health,” WT/MIN(01)/DEC/2,20 November 2001. http://www.wto.org/english/thewto_e/minist_e/min01/mindecl)trips_e.htm WTO, Brazil, India, Pakistan, Peru, Thailand and Venezuela, 2004. “Elements of the Obligation to Disclose the Source and Country of Origin of Biological Resources and/or Traditional Knowledge used in an Invention”, WTO document. IP/C/W/429, September 21 WTO Council on TRIPS, 2003. “WTO Decision on Implementation of Paragraph 6 of the Doha Declaration on the TRIPS Agreement and Public Health” IP/C/405. WTO, 2003. “Implementation of Paragraph 6 of the Doha declaration on the TRIPS Agreement and Public Health” September 2. WT/L/540 http://docsonline.wto.org WTO, 2005. “Compulsory Licensing of Pharmaceuticals and TRIPS” October. TRIPS and Health: FAQs. http://www.wto.org/english/tratop_e/trips_e/public_health_faq_e.htm WTO, 2005. “Legal Arguments to Support the African Group Proposal on the Implementation of Paragraph 11 of the 30 August 2003 Decision” March 1. IP/C/W/440. Yu, Peter, 2004. “The Escalating Copyright Wars,” Hoftsra Law Review 32: 907-951. Zald, Mayer. 1996. “Culture, Ideology and Strategic Framing” in Doug McAdam et al. eds. Comparative Perspectives on Social Movements: Political Opportunities, Mobilizing Structures and Cultural Framings. Cambridge: Cambridge University Press.