Unit 1 - OrgSites
advertisement

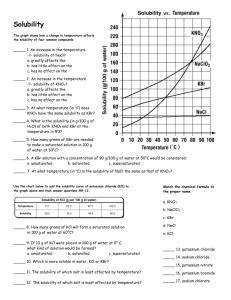
Name________________________________________ Date______________ Block_______ 1. Distinguish between indirect and direct water uses. Give an example of each. ________________________________________________________________________________________ ________________________________________________________________________________________ ________________________________________________________________________________________ 2. Why is water purification necessary? ________________________________________________________________________________________ ________________________________________________________________________________________ ________________________________________________________________________________________ 3. How do charcoal adsorption and gravel/sand filtration differ in terms of what they remove from water? ________________________________________________________________________________________ ________________________________________________________________________________________ ________________________________________________________________________________________ 4. Identify the three largest storage reservoirs for Earth’s water supply. ________________________________________________________________________________________ 5. Find out what grey water is and give a definition. Then tell why some communities use a certain amount of grey water to meet their water supply needs? _______________________________________________________________________________________ ________________________________________________________________________________________ ________________________________________________________________________________________ 6. Name two ways that a family can most effectively conserve water. ________________________________________________________________________________________ ________________________________________________________________________________________ ________________________________________________________________________________________ 7. Why are the issues of water conservation and water purification important to the discussion of the Riverwood fish kill crisis? ________________________________________________________________________________________ ________________________________________________________________________________________ ________________________________________________________________________________________ 8. On the back of this paper draw a model of a solution in which water (H 2O) is the solvent and carbon dioxide gas (CO2) is the solute. 9. Classify each of the following as an element or a compound. a. NaCl ___________________________ b. C ___________________________________ c. CO ___________________________ d. KNO3 _______________________________ 10. On the back of this paper draw a model of a molecular compound made up of 2 atoms of B and 2 atoms of E. 11. Complete the following table by naming the elements present and the number of each type of atom. Compound Element Names Number of Atoms of Each Element CO2 CaCl2 Al2S3 NH4NO3 12. Write a chemical equation that represents the following word equation: One molecule of nitrogen (N2) reacts with 3 molecules of hydrogen (H 2) to form 2 molecules of ammonia (NH3). _________________________________________________________________________________ 13. Differentiate between protons and electrons in terms of their relative charges. ________________________________________________________________________________________ ________________________________________________________________________________________ 14. Decide whether each of the following atoms is electrically neutral. Element Protons Electrons Neutral? Gold 79 76 Helium 2 2 Fluorine 9 10 15. Classify the following as anions, cations, or electrically neutral atoms. a. K+ _____________ b. Ag _____________ c. N3- _____________ d. Ba2+ ___________ 16. Write the symbol and show the electrical charge (if any) on the following atoms or ions: a. helium with one proton and one electron _____________ b. lithium with three protons and two electrons _____________ c. fluorine with nine protons and ten electrons _____________ 17. Complete the following table by writing the name and formula for the compound formed from the following ions. Cation Anion Formula Name Mg2+ O2Al3+ Cl- Mg2+ NO3- NH4+ CO32- C.1 Supplement: Solubility and Solubility Curves Using the solubility curves found in Figure 1.33 (page 45 of your textbook), solve the following problems. 1. What is the solubility of potassium nitrate (KNO3) in 100.0 g of water at 80.0 °C? _____________ 2. What is the solubility of potassium chloride (KCl) in 100.0 g of water at 50.0 °C? _____________ 3. What is the solubility of sodium chloride (NaCl) in 100.0 g of water at 90.0 °C? _____________ 4. What is the minimum temperature needed to dissolve 180.0 g of KNO3 in 100.0 g of water? _____________ 5. What is the minimum temperature needed to dissolve 35.0 g of KCl in 100.0 g of water? _____________ 6. At what temperature do KCl and KNO3 have the same solubility? _____________ 7. How much more KCl will dissolve at 90.0 °C than at 20.0 °C? _____________ 8. If 50.0 g of NaCl is mixed with 100.0 g of water at 80.0 °C, how much will not dissolve? _____________ 9. If 15.0 g of KCl is added to 100.0 g of water at 30.0 °C, how much more must be added to saturate the solution? _____________ 10. Classify as saturated or unsaturated a solution that contains 90.0 g of KNO 3 in 100.0 g of water at 60.0 °C. ________________________ 11. Classify as saturated or unsaturated a solution that contains 50.0 g of KCl in 100.0 g of water at 70.0 °C. _______________________