EffectofTemponSolubilityofSaltGCCEGLXTN
advertisement

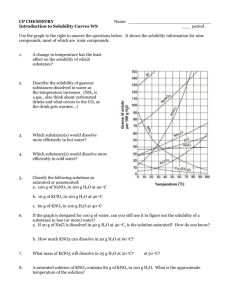
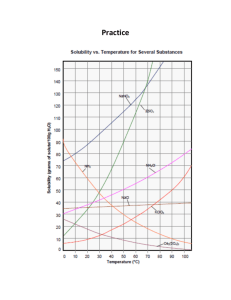
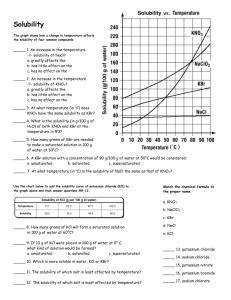
1.1, 1.2 Effect of Temperature on Solubility of a Salt-Teachers Notes COS Objectives: Standard 4: Solubility Standard 4: Bullet 1: Solubility Curves Standard 4: Bullet 4: Rate of solution ENGAGE Super Saturation Teacher Notes COS Objectives: Standard 4: Solubility Standard 4: Bullet 1: Solubility Curves Standard 4: Bullet 4: Rate of solution ENGAGE Demonstration of rapid precipitation from a Reusable Hand Warmer (1) Explain the concepts of saturation, supersaturation, crystallization, and seed crystals (2) Hand each pair of students one of the Reusable Hand Warmers. (3) Have one student Flex the metal disc back and forth until crystals start to form. Once crystals start to farm gently knead the pouch to intensify the heat. Materials for Engagement activity Reusable Hand Warmer Hot Plate Large Beaker Water Tongs NASA and Auburn University Global Climate Change Education Chemistry Module Page 18 Restoring to liquid form (using a hotplate) (1) In a large beaker boil water. (2) Hold the Reusable Hand Warmer with the Tongs in the boiling water (be sure to not let it touch the bottom of the beaker or the plastic will melt! If you get tired of holding the Reusable Hand Warmer with the Tongs, an alternative is to wrap it in a heavy cloth towel. The cloth towel should protect the plastic from melting against the bottom of the beaker. Then you can let the protected (cloth wrapped) Reusable Hand Warmer drop to the bottom of the beaker. (3) Remove the Reusable Hand Warmer when ALL of the crystals are gone (approx. 7-10 minutes) (4) Let it cool before use (the plastic is wearer when hot and the metal disc may slice it open). NOTE: If you want to use a microwave to Restore the Hand Warmer watch the video at: http://beprepared.com/article.asp?ai=725& EXPLORE See Student Handout Sample Data Solubility of KNO3 (per 100 g H2O) vs. Temperature Trial Solubility (g / 100 g H2O) Temp (°C) 1 40.0 25.5 2 80.0 47.2 3 120.0 64.0 4 160.0 76.4 NASA and Auburn University Global Climate Change Education Chemistry Module Page 19 EXPLAIN Answers to Questions 1. According to your data, how is solubility of KNO3 affected by an increase in temperature of the solvent? Solubility of KNO3 increases with increasing temperature. 2. Using your printed graph, tell if each of these solutions would be saturated or unsaturated: a. 110 g of KNO3 in 100 g of water at 40°C _____Saturated________________ b. 60 g of KNO3 in 100 g of water at 70°C _____Unsaturated______________ c. 140 g of KNO3 in 200 g of water at 60°C _____Unsaturated______________ 3. According to your graph, will 50 g of KNO3 completely dissolve in 100 g of water at 50°C? Explain. Yes, it will dissolve, since this point is below the solubility curve. 4. According to your graph, will 80 g of KNO3 completely dissolve in 100 g of water at 30°C? Explain. No, it will not dissolve, since this point is above the solubility curve. 5. At 30°C, approximately how many grams of KNO3 would dissolve in 100 g of water? At 30°C, ~50 g of KNO3 would dissolve in 100 g of water. EXTEND 1. One way to extend this is to have students use the following graph to make comparisons between different salts or between salts and the gas SO2. NASA and Auburn University Global Climate Change Education Chemistry Module Page 20 Note that the solubility of the gas decreases with an increase in temperature which is opposite that of the general trend for salts. You can ask students to figure out how much more solution NaCl is in boiling water than room temperature water and compare that to KCl. Then ask the students why do they think we used KCl in the experiment? Answer: It’s solubility changes considerably more than salt in the temperature range that we use in the lab. NASA and Auburn University Global Climate Change Education Chemistry Module Page 21 2. Visit NASA’s website http://nasascience.nasa.gov/earth-science/oceanography/physicalocean/salinity. Answer the following questions: Where are the highest salinity and the lowest salinity of oceans found? Highest salinity of seawater is in the mid-Atlantic Ocean Lowest salinity is found near the Arctic and Antarctic Why higher seawater salinity is found in the middle of oceans near the equator? High salinity is usually in the center of the ocean basins away from the mouths of rivers, which input fresh water. High salinity near equator is due to intensive evaporation. Why lower seawater salinity is found near the Arctic and Antarctic areas? Due to glaciers melting Describe two techniques used for measuring ocean water salinity. Which method can provide a complete global data with relatively short periods (a few days) of time? Salinity data can be collected by satellites (using radiometers) or in situ measurement (by ships, buoys, drifters, and moorings). Satellite data collection covers the entire globe and can be done in a few days. Troubleshooting & Safety 1. Have 20 g of solid KNO3 available per student lab team. HAZARD ALERT: Potassium nitrate is a strong oxidant; fire and explosion risk when heated or in contact with organic material; skin irritant. Hazard Code: B—Hazardous. The hazard information reference is: Flinn Scientific, Inc., Chemical & Biological Catalog Reference Manual, 2000, P.O. Box 219, Batavia, IL 60510. See Appendix F of this book, Chemistry with Calculators, for more information. 2. Students should be well-prepared for this experiment in order to comfortably complete it during a 50-minute class period. Remind them of the tips in Step 11 of the student procedure. Starting hotwater baths promptly will save time. The 2-g and 4-g samples do not need to be heated above 60°C in order to have all of the solid dissolve; thus, the water bath does not need to be at 90°C for these two trials. Toward the end of the class period, some students may have to use cool-water baths, especially for the 2-g and 4-g samples. 3. When using hot plates, caution students to keep the temperature probe wire well away from the hot plate, as mentioned in Step 8. 4. The Vernier temperature calibrations that are stored in the DataMate data-collection program will work fine for this experiment. NASA and Auburn University Global Climate Change Education Chemistry Module Page 22