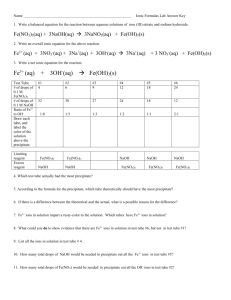
Lab 19 (Reactions of Aqueous Ionic Compounds)
advertisement
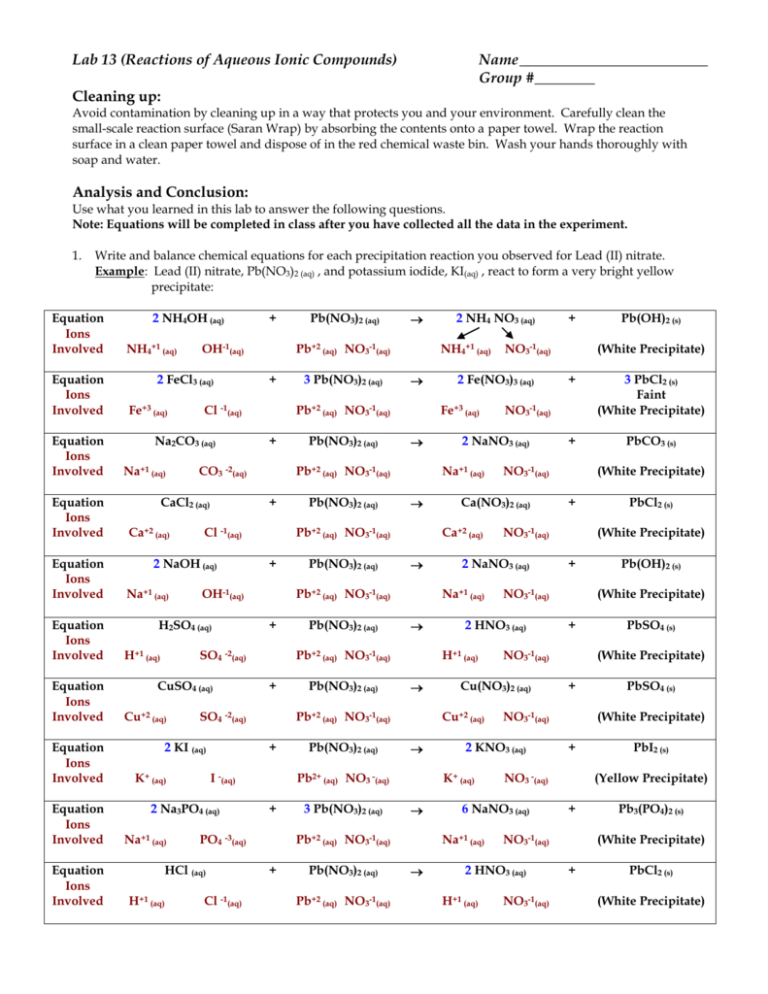
Lab 13 (Reactions of Aqueous Ionic Compounds) Name _________________________ Group #________ Cleaning up: Avoid contamination by cleaning up in a way that protects you and your environment. Carefully clean the small-scale reaction surface (Saran Wrap) by absorbing the contents onto a paper towel. Wrap the reaction surface in a clean paper towel and dispose of in the red chemical waste bin. Wash your hands thoroughly with soap and water. Analysis and Conclusion: Use what you learned in this lab to answer the following questions. Note: Equations will be completed in class after you have collected all the data in the experiment. 1. Write and balance chemical equations for each precipitation reaction you observed for Lead (II) nitrate. Example: Lead (II) nitrate, Pb(NO3)2 (aq) , and potassium iodide, KI(aq) , react to form a very bright yellow precipitate: Equation Ions Involved Equation Ions Involved Equation Ions Involved Equation Ions Involved Equation Ions Involved Equation Ions Involved Equation Ions Involved Equation Ions Involved Equation Ions Involved Equation Ions Involved 2 NH4OH (aq) NH4+1 (aq) OH-1(aq) 2 FeCl3 (aq) Fe+3 (aq) Ca+2 (aq) H+1 (aq) + + + K+ (aq) Na+1 (aq) Pb(NO3)2 (aq) Pb(NO3)2 (aq) Pb(NO3)2 (aq) Pb(NO3)2 (aq) + 3 Pb(NO3)2 (aq) Pb+2 (aq) NO3-1(aq) + Pb(NO3)2 (aq) Pb+2 (aq) NO3-1(aq) NO3-1(aq) PbCl2 (s) (White Precipitate) + Pb(OH)2 (s) (White Precipitate) + PbSO4 (s) (White Precipitate) + PbSO4 (s) (White Precipitate) + PbI2 (s) (Yellow Precipitate) + NO3-1(aq) 2 HNO3 (aq) H+1 (aq) + NO3 -(aq) 6 NaNO3 (aq) Na+1 (aq) (White Precipitate) NO3-1(aq) 2 KNO3 (aq) K+ (aq) PbCO3 (s) NO3-1(aq) Cu(NO3)2 (aq) Cu+2 (aq) + NO3-1(aq) 2 HNO3 (aq) H+1 (aq) 3 PbCl2 (s) Faint (White Precipitate) NO3-1(aq) 2 NaNO3 (aq) Na+1 (aq) + NO3-1(aq) Ca(NO3)2 (aq) Ca+2 (aq) Pb2+ (aq) NO3 -(aq) PO4 -3(aq) Cl -1(aq) Pb(NO3)2 (aq) Pb(OH)2 (s) (White Precipitate) NO3-1(aq) 2 NaNO3 (aq) Na+1 (aq) Pb+2 (aq) NO3-1(aq) I -(aq) HCl (aq) H+1 (aq) + + 2 Na3PO4 (aq) Pb+2 (aq) NO3-1(aq) SO4 -2(aq) 2 KI (aq) Pb(NO3)2 (aq) + NO3-1(aq) 2 Fe(NO3)3 (aq) Fe+3 (aq) Pb+2 (aq) NO3-1(aq) SO4 -2(aq) Cu+2 (aq) Pb+2 (aq) NO3-1(aq) OH-1(aq) CuSO4 (aq) 3 Pb(NO3)2 (aq) 2 NH4 NO3 (aq) NH4+1 (aq) Pb+2 (aq) NO3-1(aq) Cl -1(aq) H2SO4 (aq) Pb+2 (aq) NO3-1(aq) + 2 NaOH (aq) Na+1 (aq) + CO3 -2(aq) CaCl2 (aq) Pb(NO3)2 (aq) Pb+2 (aq) NO3-1(aq) Cl -1(aq) Na2CO3 (aq) Na+1 (aq) + Pb3(PO4)2 (s) (White Precipitate) + PbCl2 (s) (White Precipitate) 2. You may have noticed that some chemicals formed precipitates when mixed with ammonia, NH 3. This is because ammonia reacts with water to produce ammonium hydroxide, NH 4OH (aq). Equation NH3 (g) + H2O (l) NH4OH (aq) To write the chemical equation for the precipitates when mixed with ammonia, you need to replace NH 3 with ammonium hydroxide, NH4OH (aq). Example: Equation Ions Involved Equation Ions Involved Equation Ions Involved Equation Ions Involved 3. NH4+1 (aq) + OH-1(aq) Pb+2 (aq) NO3-1(aq) 2 NH4OH (aq) NH4+1 (aq) + OH-1(aq) + OH-1(aq) AgNO3 (aq) + OH-1(aq) Fe+3 (aq) Cl -1(aq) Pb(OH)2 (s) NO3-1(aq) (White Precipitate) + Cu(OH)2 (s) SO4-2(aq) NH4NO3 (aq) (Blue Precipitate) + AgOH (s) NH4+1 (aq) NO3-1(aq) FeCl3 (aq) + (NH4)2SO4 (aq) NH4+1 (aq) Ag+1 (aq) NO3-1(aq) 3 NH4OH (aq) NH4+1 (aq) CuSO4 (aq) 2 NH4 NO3 (aq) NH4+1 (aq) Cu+2 (aq) SO4-2(aq) NH4OH (aq) NH4+1 (aq) Pb(NO3)2 (aq) 3 NH4Cl (aq) NH4+1 (aq) (Light White ppt.) + Cl -1(aq) Fe(OH)3 (s) (Orange Precipitate) Write chemical equations to describe all the reactions that produced bubbles. Equation Ions Involved Equation Ions Involved Equation Ions Involved 4. 2 NH4OH (aq) Na2CO3 (aq) Na+1 (aq) CO3 -2(aq) Na2CO3 (aq) Na+1 (aq) CO3 -2(aq) 2 HCl (aq) H+1 (aq) + CO3 -2(aq) Na2CO3 (aq) Na+1 (aq) + + Cl -1(aq) H2SO4 (aq) H+1 (aq) 2 NaCl (aq) Na+1 (aq) SO4-2(aq) 2 HNO3 (aq) H+1 (aq) Cl-1(aq) Na2SO4 (aq) Na+1(aq) SO4-2(aq) NO3-1(aq) 2 NaNO3 (aq) Na+1(aq) NO3-1(aq) + CO2 (g) + Carbon Dioxide Gas + CO2 (g) + (Water) Carbon Dioxide Gas + CO2 (g) + (Water) Carbon Dioxide Gas (Water) What chemicals do all the reactions that produced bubbles have in common? Sodium carbonate produced bubbles with all three acids. Formula: Na2CO3 (aq) 5. Which reactions gave color changes but no precipitate? Why do you think this happened? Reactants: KI(aq) + FeCl3 (aq) produced an amber colored solution. Iron (III) formed a complex ion with iodide. Complex ions stay dissolved but give color changes. 6. Which mixings could you have predicted in advance would not result in a reaction? Any combination that had common cations or anions. Examples: HNO3(aq) + Pb(NO3)2 (aq) HNO3(aq) + HCl(aq) H2O (l) both have the anion NO3-1 (aq) other anions, OH-,Cl-, SO42both have the cation H+1 (aq) other cations, Na+ H2O (l) H2O (l)