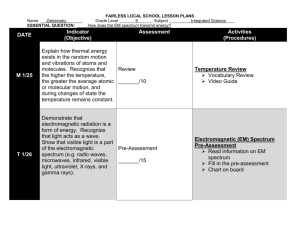
Using the Electromagnetic Spectrum Lab
advertisement
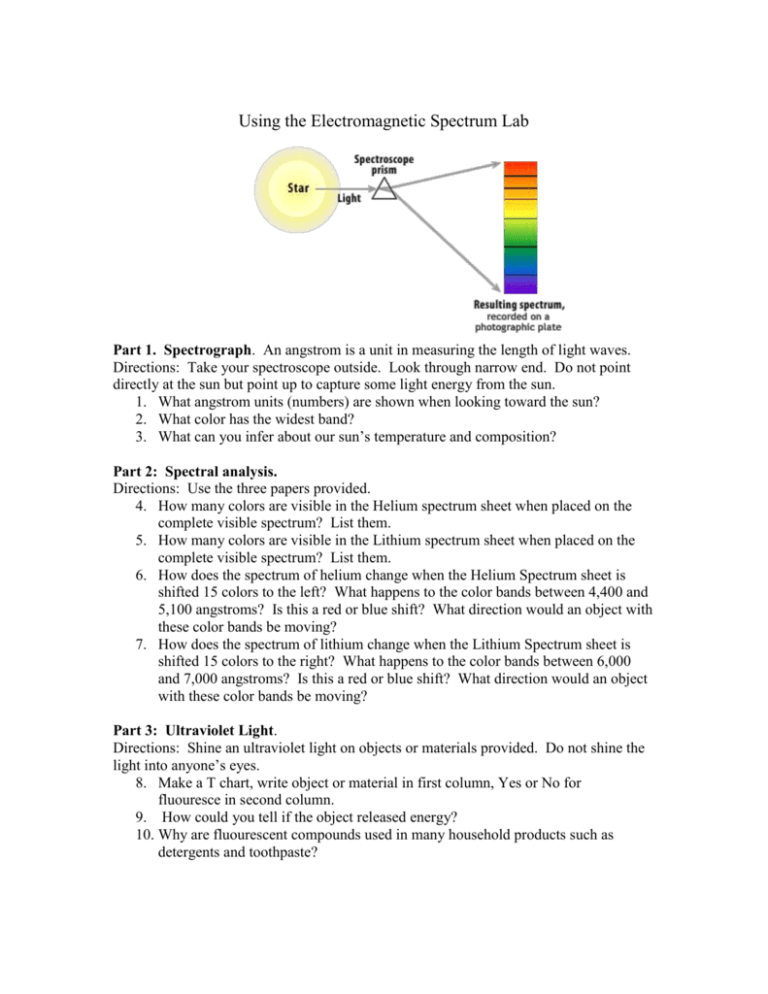
Using the Electromagnetic Spectrum Lab Part 1. Spectrograph. An angstrom is a unit in measuring the length of light waves. Directions: Take your spectroscope outside. Look through narrow end. Do not point directly at the sun but point up to capture some light energy from the sun. 1. What angstrom units (numbers) are shown when looking toward the sun? 2. What color has the widest band? 3. What can you infer about our sun’s temperature and composition? Part 2: Spectral analysis. Directions: Use the three papers provided. 4. How many colors are visible in the Helium spectrum sheet when placed on the complete visible spectrum? List them. 5. How many colors are visible in the Lithium spectrum sheet when placed on the complete visible spectrum? List them. 6. How does the spectrum of helium change when the Helium Spectrum sheet is shifted 15 colors to the left? What happens to the color bands between 4,400 and 5,100 angstroms? Is this a red or blue shift? What direction would an object with these color bands be moving? 7. How does the spectrum of lithium change when the Lithium Spectrum sheet is shifted 15 colors to the right? What happens to the color bands between 6,000 and 7,000 angstroms? Is this a red or blue shift? What direction would an object with these color bands be moving? Part 3: Ultraviolet Light. Directions: Shine an ultraviolet light on objects or materials provided. Do not shine the light into anyone’s eyes. 8. Make a T chart, write object or material in first column, Yes or No for fluouresce in second column. 9. How could you tell if the object released energy? 10. Why are fluourescent compounds used in many household products such as detergents and toothpaste?