Mapping the Essential Bipartite Nuclear Localization Signal
advertisement

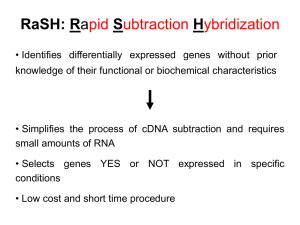
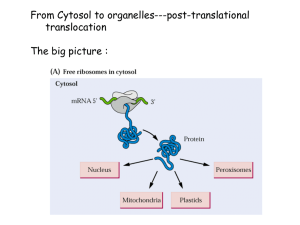
78132 Mapping the Essential Bipartite Nuclear Localization Signal Domain of UOL Protein Presenter: Gwendolyn Le Mentor: Guey-Chuen Perng During the course of herpes simplex virus type 1 (HSV-1) infection, viral proteins shuttle in and out of the cell organelles, such as the nucleus, playing a crucial regulatory role in infected cells. For a protein to be localized in the nucleus, a nuclear localization signal sequence (NLS) is required to fulfill the task. UOL, a newly identified gene from HSV-1 viral genome, contains two bipartite NLS. My goal is to check whether these NLS in the UOL protein are functional. If functional, I will further characterize which one of the two NLS' is (are) essential for the UOL protein in targeting the nucleus. Preliminary results, using a red fluorescent protein (RFP) indicator gene fused to UOL, indicated that the NLS in UOL protein is functional in transient transfected cells. I continued onto mapping the NLS domain. In this process, five sets of overlapping PCR primers attached with universal restriction enzyme sites, EcoRI and BamHI at 5' and 3' ends respectively, were designed to facilitate the directional cloning. The amplified PCR fragments were then fused in frame to the RFP gene, which will show a visible red signal. In my research so far, I have obtained three sets of fusion clones into pDs-Red vector and am currently performing transient transfection assays to check for the organelle localization of RFP signal. I have also cloned the remaining two sets into the pGEM-T vector and am currently cloning them into the pDs-Red vector to further perform the transient transfection assays.