biochem for genetics notes
advertisement

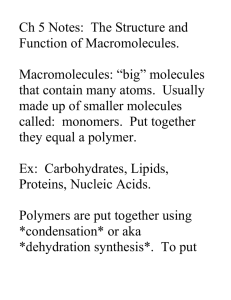
Biochemistry Notes Organic compounds: Contain carbon Originally from living or once living things In addition to carbon, most contain Hydrogen and oxygen Other elements include: Nitrogen, Sulfur, Phosphorus, Iron Calcium, sodium, chlorine, magnesium, potassium Inorganic compounds: Do NOT contain carbon CO2 and carbonate compounds, although found in living things, are not considered organic. Water: Most important inorganic compound in living things Ionic bonds: Transfer of electrons Covalent bonds: Share electrons Polar covalent bonds: Electrons “shared” unequally due to electro negativity of an atom Hydrogen Bonds: Formed between hydrogen in one molecule and an electro-negative molecule in another 1/10th strength of covalent bond Very important in interactions of biomolecules Primary force in way proteins fold and DNA bases bond Structure & types of organic compounds. Major difference between organic and inorganic compounds Greater size and complexity because of carbon’s electron structure Carbohydrates: Main source of energy Made of elements carbon, hydrogen, oxygen ratio of hydrogen to oxygen is 2 to 1, like water H2O example: C6H12O6 common monosaccharides Many types of carbohydrates: Sugars: names of sugars end in ~ose (glucose, fructose, dextrose) Monosaccharides : simple sugars C6H12O6 or C5H10O5 Monomer: small repeating unit that makes up a polymer, monosaccharide or amino acid Polymer: large molecule made up of many small repeating units Dehydration synthesis A reaction in which two molecules are bonded together by the removal of a water molecule dehydration: removing water synthesis: put together formation of a disaccharide: Hydrolysis: Process by which large molecules are broken apart by the addition of water molecules (requires enzymes, too) Hydro: water Lysis: break down or apart Disaccharides: Formed by joining two simple sugars Sucrose or table sugar is a disaccharide made of glucose and fructose Lactose, a sugar found in milk, is made of glucose and galactose Lactose intolerant: inability to digest, hydrolyze, lactose into galactose and glucose; not enough of the enzyme lactase Polysaccharides: Polymer of many repeating sugar units Starch: sugar is stored in plants as starch; found in seeds, roots, and stems Glycogen: surplus sugar stored in the liver Cellulose: found in plants, humans cannot digest cellulose—called fiber some animals, like rabbits, can digest cellulose using appendix Lipids: includes fats, oils, waxes Made of carbon, hydrogen, oxygen like carbohydrates, however, much less oxygen insoluble in water – hydrophobic, “water fearing Triglycerides: Nutritionally and medically important Energy storage Oils: liquid at room temperature; come from Plants: corn oil, olive oil Fats: solid to semisolid at room temp; from animals store fat: prevents heat loss; cushions certain organs Saturated and unsaturated fats: Saturated: all carbon to carbon bonds are single bonds Unsaturated: one or more carbon pairs are joined by a double or triple bond c-c-c-c-c-c-c-c c=c-c-c-cpolyunsaturated: more than one double or triple bond Hydrogenated: process used to turn unsaturated fats into saturated fats Phospholipids: Found primarily in cell membrane Head---hydrophyllic—literally water loving Tail—hydrophobic—water fearing Steroids: Composed of three 6-carbon rings bound to a 5-carbon ring Hormones, venoms, pigments cholesterol: Soft, waxy fat found in cell membrane Part of some hormones Too much cholesterol can contribute to cardiovascular disease Proteins made of the elements carbon, hydrogen, and oxygen like carbohydrates and lipids, but also contain nitrogen and sometimes sulfur and phosphorous Proteins perform many jobs Structural-ex. Collagen (part of bone) Enzyme-ex. Lactase Transport-ex. hemoglobin Contractile-ex. myosin-muscle contraction Hormone-ex. Insulin Antibody-ex. gamma globulin Pigment-ex. Melanin Recognition- ex. Cell surface Toxins- ex. Botulism toxin (botox) Protein are made of amino acids 20 amino acids, but they combine to form 1000’s of proteins Two amino acids combine with a special bond called a peptide bond The resulting molecule is called a dipeptide A chain of amino acids connected by peptide bonds is called a polypeptide Proteins are made of one or more of these polypeptides, containing anywhere from 50 to 100,000 amino acids. Structure of proteins Primary: the “strand” of amino acids Secondary: the chain of amino acids twists or bends to form a more complex structure, usually a coil or a “pleat Tertiary: the coil or pleat fold to make a ball Quaternary: two or more chains fold together Nucleic Acids Made of carbon, hydrogen, oxygen, phosphorous, and nitrogen Two kinds DNA or deoxyribonucleic acid Hereditary material passed from one generation to the next Genes: sequence of nucleotides of DNA that codes for a particular polypeptide RNA or ribonucleic acid Three kinds: rRNA, tRNA and mRNA RNA is very important Necessary to “unlock” DNA Necessary to make proteins Structure of nucleic acids A backbone of 5 carbon sugars (ribose or deoxyribose) bonded to a phosphate group (PO4) Attached to one of four nitorgenous bases: adenine, guanine, cytosine,or thymine (uracil in RNA) In DNA there are two backbones and the bases align with each other The “ladder” coils into the familiar double helix