Solution Concentrati..
advertisement
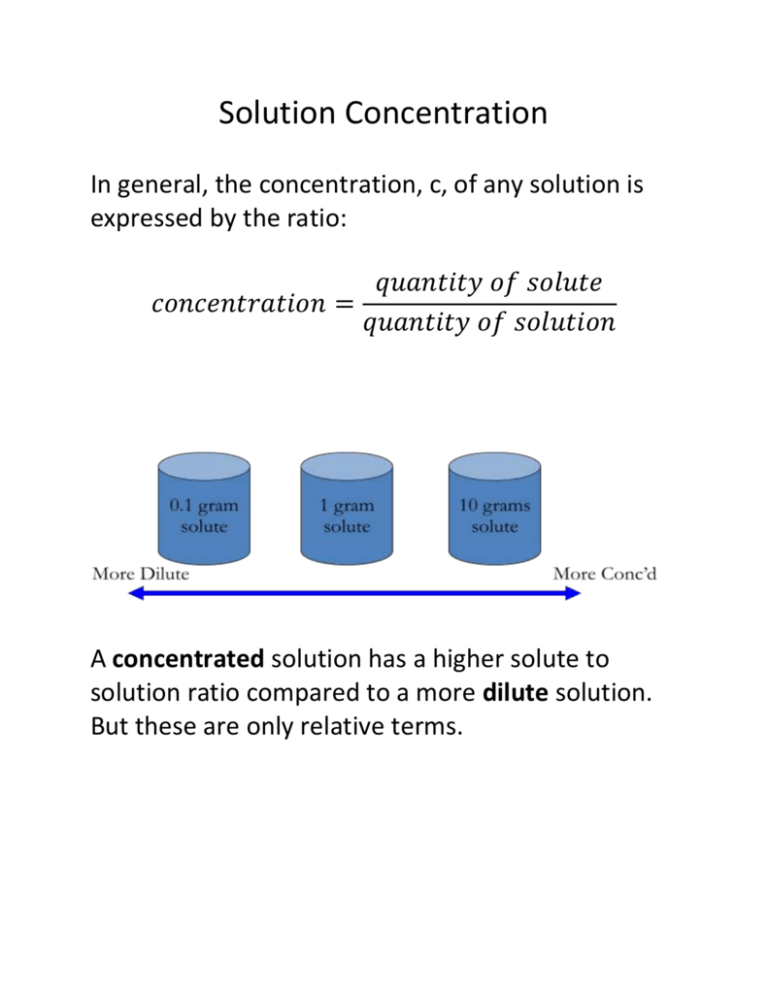
Solution Concentration In general, the concentration, c, of any solution is expressed by the ratio: 𝑞𝑢𝑎𝑛𝑡𝑖𝑡𝑦 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 = 𝑞𝑢𝑎𝑛𝑡𝑖𝑡𝑦 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 A concentrated solution has a higher solute to solution ratio compared to a more dilute solution. But these are only relative terms. Percentage Concentration 3 ways to express percentage concentration: -Percentage volume by volume (% V/V) -Percentage weight by volume (%W/V) -Percentage weight by weight (%W/W) #1 “percentage volume by volume” “% V/V” Vsolute c 100% Vsolution -Usually used to express concentrations of liquids dissolved in liquids. #2 “percentage weight by volume” “% W/V” msolute c 100% Vsolution -mass must be in grams -volume must be in mL Example: A 250 mL bottle of isopropyl alcohol is advertised as being 70% W/V. What is the mass of alcohol in the bottle? #3 “percentage weight by weight” “% W/W” msolute c 100% msolution -Can use any unit of mass, but must be the same on the top and bottom. Parts per million concentration (ppm) For very dilute solutions, we use a special ratio called parts per million (ppm). A concentration of 1 ppm means there is a million times more solution than solute (imagine 1 drop in a bathtub full of water). 1 ppm = 1 mg/kg = 1 mg/L = 1 g/106 mL Amount concentration -Since chemical reactions are represented by moles, it is useful to represent concentration in moles as well. This is the “amount concentration”. n c V -Units: mol/L or M -can be indicated by putting square brackets around a chemical. e.g. [NaOH(aq)]=1.5 mol/L “the amount concentration of sodium hydroxide in water is 1.5 moles per litre” Percentage Volume by Volume (% V/V) Vsolute c 100% Vsolution Percentage Weight by Volume (% W/V) msolute c 100% Vsolution Percentage Weight by Weight (% W/W) msolute c 100% msolution Parts Per Million 1 ppm= 1mg/kg = 1 mg/L Amount Concentration n=amount of solute in moles V=Volume of solution in Litres n c V Review Molecular compounds -Solubility can be predicted by polarity (like dissolves like) -Do not form ions in a solution* -Do not conduct electricity* (non-electrolyte) -Do not affect litmus* -C6H12O6(s) C6H12O6(aq) *unless they are an acid Ionic Compounds -Solubility can be predicted using solubility chart -Dissociate to form ions in a solution -Conduct electricity in solution (electrolyte) -Do not affect litmus paper* *unless they are a base -LiBr(s) Li+(aq) + Br-(aq) Acids -Molecular compounds -Ionize to form ions in a solution -Cation is H+(aq) -Conduct electricity (electrolyte) -Turn litmus paper red -HNO3(s) H+(aq) + NO3-(aq) Bases -Ionic compounds -Dissociate to form ions in a solution -Anion is OH-(aq) -Conduct electricity (electrolyte) -Turn litmus paper blue -KOH(s) K+(aq) + OH-(aq) Energy Changes -Breaking bonds absorbs energy (endothermic) -Forming bonds releases energy (exothermic) -Both occur during dissolving, but the net energy change determines if it is exothermic or endothermic