Chapter 3 Timeline Project
advertisement

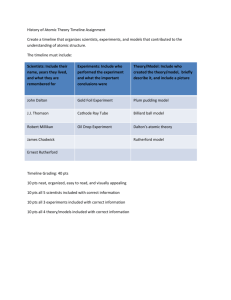
Name Class Date Skills Worksheet Reinforcement Atomic Timeline If you were asked to draw the structure of an atom, what would you draw? Throughout history scientists have accepted five atomic models. Our perception of the atom has changed from the early Greek model because of clues or evidences that have been gathered through scientific experiments. As more evidence was gathered old models were discarded or improved upon. Your goal is to trace the atomic theory through history. You and your partner will use the sources provided (textbook and internet) to develop a timeline that outlines the key scientists and experiments associated with the development of modern atomic theory. The timeline should include the names of the scientists, a description of the accomplishments, pictures of experimental equipment or atomic models, and description. The table below contains a number of statements connected to major discoveries in the development of atomic theory. These must be included in your timeline 1. In each box, write the name of the scientist(s) associated with the statement. Choose from among the following scientists: Democritus, Rutherford, Thomson, Dalton, Bohr, Schrödinger, and Heisenberg. 2. On a separate sheet of paper, construct a timeline, and label the following: 440 BCE, 1803, 1897, 1909–1911, 1913, and the twentieth century. Cut out the boxes and tape or glue each box at the correct point along the timeline. A. Most of an atom’s mass is in the nucleus. B. There is a small, dense, positively charged nucleus. C. There are small, negatively charged particles inside an atom. D. Electrons can jump from a path in one level to a path in another level. E. Atoms of different elements are different. F. He conducted the cathode-ray tube experiment. G. Atoms are small, hard particles. H. Atoms contain mostly empty space. I. Atoms are “uncuttable.” J. He conducted experiments in combining elements. K. Electrons travel in certain paths, or energy levels. L. Electron paths cannot be predicted. M. His theory of atomic structure led to the “plum-pudding” model. N. His model had electrons surrounding the nucleus at a distance. O. Atoms of the same element are exactly alike. P. Electrons are found in electron clouds, not paths. Q. All substances are made of atoms. R. Atoms are made of a single material formed into different shapes and sizes. S. He conducted the gold foil experiment. T. He wanted to know why elements combine in specific proportions. 3. Now find additional 5 facts (total should be minimum of 25) to add to the ones given. These 5 facts must be from scientists that are NOT already named. Your timeline should be on a poster paper (will be provided) with pictures and be legible! This assignment will count as a test grade, so take your time and do a good job! You will be given 2 days in the computer lab to research material and print needed pictures. You will then be given 2 additional days in the classroom to put the timeline together. DUE DATE: ____________________ (Cut off rubric and hand in with completed timeline) ----------------------------------------------------------------------------------------------------------------Names: ____________________________ Class: ___________ ____________________________ Atomic Structure Timeline Rubric Timeline Poster/Pamphlet 25 Fact Entries 50 pts _______ 10 Required Scientist 20 pts _______ Pictures (min. of 5) 10 pts _______ Grammar & Spelling 5 pts _______ Creativity/Neatness 5pts _______ Total: 90 pts _______