WS ONE
advertisement
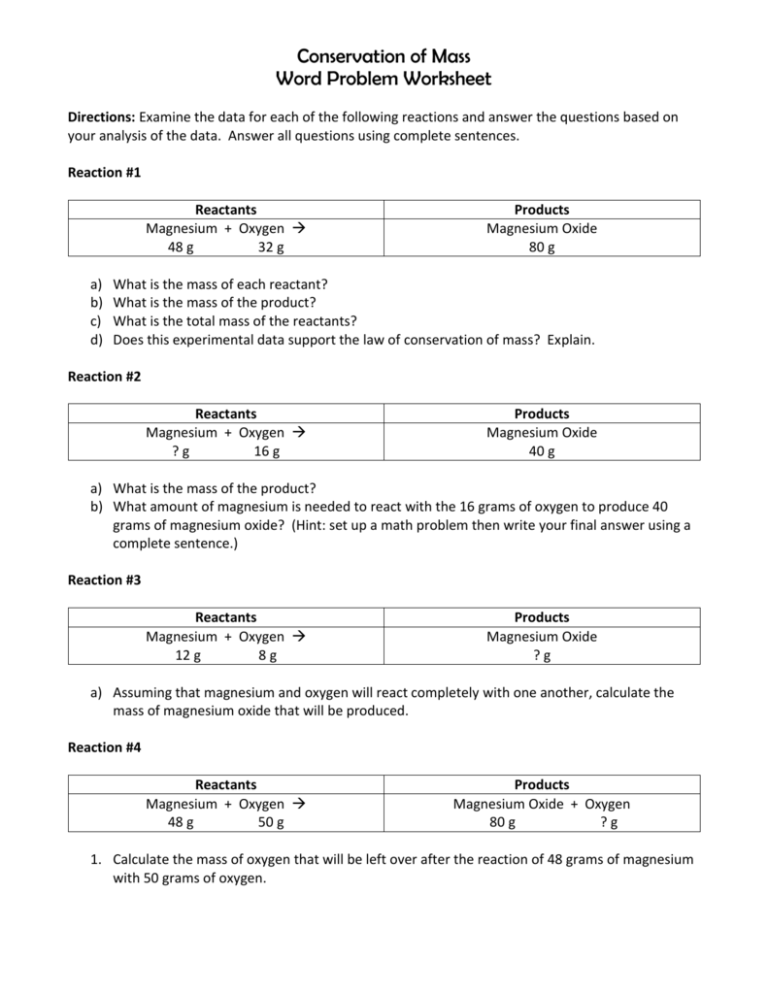
Conservation of Mass Word Problem Worksheet Directions: Examine the data for each of the following reactions and answer the questions based on your analysis of the data. Answer all questions using complete sentences. Reaction #1 Reactants Magnesium + Oxygen 48 g 32 g a) b) c) d) Products Magnesium Oxide 80 g What is the mass of each reactant? What is the mass of the product? What is the total mass of the reactants? Does this experimental data support the law of conservation of mass? Explain. Reaction #2 Reactants Magnesium + Oxygen ?g 16 g Products Magnesium Oxide 40 g a) What is the mass of the product? b) What amount of magnesium is needed to react with the 16 grams of oxygen to produce 40 grams of magnesium oxide? (Hint: set up a math problem then write your final answer using a complete sentence.) Reaction #3 Reactants Magnesium + Oxygen 12 g 8g Products Magnesium Oxide ?g a) Assuming that magnesium and oxygen will react completely with one another, calculate the mass of magnesium oxide that will be produced. Reaction #4 Reactants Magnesium + Oxygen 48 g 50 g Products Magnesium Oxide + Oxygen 80 g ?g 1. Calculate the mass of oxygen that will be left over after the reaction of 48 grams of magnesium with 50 grams of oxygen. Balanced or Not? Directions: For each equation, count the number of atoms of each element on the reactant side. Then count the number of atoms of each element on the product side. Next, determine if the equation is balanced or not. Using complete sentences, briefly explain your answer. 1. 2H2 + O2 2H2O 2. N2 + H2 NH3 3. S8 + O2 8SO3 4. 2HgO 2Hg + O2 5. CO2 + H2O C6H12O6 + O2 6. Zn + 2HCl ZnCl2 + H2 7. SiCl4 + 4H2O H4SiO4 + HCl 8. C6H12O6 + 6O2 6CO2 + 6H2O 9. Na + H2O NaOH + H2 10.C10H16 + Cl2 C + HCl Balanced or Not? Directions: For each equation, count the number of atoms of each element on the reactant side. Then count the number of atoms of each element on the product side. Next, determine if the equation is balanced or not. Using complete sentences, briefly explain your answer. 1. N2 + H2 NH3 2. S8 + O2 8SO3 3. 2HgO 2Hg + O2 4. CO2 + H2O C6H12O6 + O2 5. Zn + 2HCl ZnCl2 + H2 6. SiCl4 + 4H2O H4SiO4 + HCl 7. C6H12O6 + 6O2 6CO2 + 6H2O 8. Na + H2O NaOH + H2 Directions: For each equation, choose the answer choice that gives the missing coefficient that would correctly balance the equation. Write a short explanation of your choice 1. N2 + ?H2 2NH3 a. 2 b. 3 c. 6 2. S8 + ?O2 8SO3 a. 8 b. 12 c. 6 3. ?HgO 2Hg + O2 a. 2 b. 1 c. 4 4. SiCl4 + 4H2O H4SiO4 + ?HCl a. 2 b. 8 c. 4 5. 4Fe + ?O2 2Fe2O3 a. 2 b. 3 c. 4





