Instruction Set Final Draft
advertisement

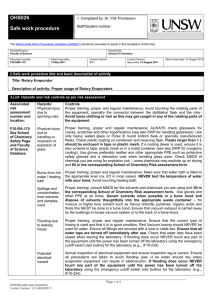
Alexander Shumski How to Operate a Rotary Evaporator What is a Rotary Evaporator? A rotary evaporator or “roto-vap” (as it is commonly known) is a laboratory instrument commonly used to remove the solvent from a solution. This is achieved by exploiting the evaporation behavior of volatiles by pulling a vacuum and increasing the surface area by rapid rotation of the round bottom flask used to hold the sample. A rough visualization of one such device can be seen below. The solvent from point A is evaporated into the column at point F, and cooled by the water running through the coil, causing it to condense into the reservoir at G. Source: Wektoryzacja na podstawie rysunku wykonanego przez. 30.10. 2009, originał:12.05.2004 (Creative Commons, Polish Wikimedia Commons) What Will You Need? A sample dissolved in a volatile solvent A round bottom flask large enough to hold twice the volume of the sample solution A rotary evaporator (any brand) Access to a laboratory vacuum line A water source (any clean water will do, tap water is commonly used) Three plastic or rubber hoses (the roto-vap will usually have these already attached) A set of rotary evaporator clamps (they look like solid colored two connected half circles) Getting Started Before starting the evaporation process, make sure that you fully understand the materials you are working with. Read any available OSHA Material Safety Datasheets (MSDS), or literature on the solvents you will be removing. In general, the solvent should be volatile, but not violently reactive when heated. In the vast majority of your potential experiments the solvent is going to be easily removed, as it is in the case of most low density organics, like dichloromethane (DCM) or most laboratory ethers. While it is possible to remove heavy alcohols or even water using a rotary evaporator, you should be aware that it will take a very long time to do so. Sample Preparation It’s very easy to prepare a solution for rotary evaporation, requiring only that you know how to fill a bottle. In other words, there’s only really one step. This makes the preparation a good example of the layout of this instruction set. 1. Take your liquid sample, and transfer it into a glass round bottom flask with a volume that is at minimum twice as large as your total sample solution. (The numbered lines are the instructional steps, indicating actions you will have to perform.) a. You can do this with any technique you wish, but the most common method is either via pipette or by carefully pouring the solution from the original vessel into the flask. (Indented lines with letters indicate feedback comments, or alternative methods when available. Consider these to be “add-ons” to the general instructions.) Note: Ideally, you want to perform any product forming reactions inside a round bottom flask to avoid having to transfer it. Alexander Shumski April 8,2015 Preparing the Machine 1. Check the hose connections (small tapered glass tubes or ports), and make sure that they are securely attached. There should be two water lines that lead into the glass coil (F), and a single vacuum line attached to the central chamber of the glass chamber (H). 2. If the water basin (B) is empty, fill it with water until it is roughly half full. 3. Turn on the water, making sure that it is cleanly running through the coil and into a recirculation reservoir or sink. Keep the water running throughout the process of evaporation. 4. Make sure the vacuum line is off, and slide your sample flask onto the end of the connection point closest to the water basin, so that your bulb is either touching or above the water. Grasp the flask firmly, and place one of the connecting clips so that the flask is firmly attached to the machine. a. Caution: Make sure that you do not let go of the sample bulb until it is firmly clipped on, as it will slide off the end and fall into the bath. b. The wide end of the clamp should go onto the bulb side, and the smaller end onto the machine. c. Sometimes, there will be a glass trap bulb between (A) and (C), this just means that the connection point will be slightly further out into the bath. 5. Turn on the vacuum line and twist the stopper (H). Wait a few second, and twist it again. If you hear a high pitched whistle or rush of air, the vacuum is working correctly. Alexander Shumski April 8,2015 Running the Machine 1. Turn on the water bath by pressing the on button below the basin, setting the heat to the level recommended for your solvent. Do not set it above 40°C unless specifically instructed to, as this is usually enough to evaporate the solvent. a. The recommended temperature can be found in the user manual or on a chart provided by the manufacturer. 2. There will be a pair of buttons with down and up arrows, or a sliding button on the handle that raises and lowers the bulb. Carefully find it, and slowly lower the bulb into the water bath. 3. Turn the large dial positioned on the same arm as the height adjustment buttons, setting the speed somewhere between 40-100 rpm, so that the liquid runs slightly up the side of the bulb as it spins. a. If you have set the machine up correctly, the solution will start boiling. b. Note: Sometimes the solution does not violently boil, but you can tell that the machine is working if fluid is dripping off of the cooling coil (F) into the waste reservoir (G). 4. When the solvent stops boiling and no liquids are dripping into the waste reservoir, turn the dial to 0 rpm, and wait for the bulb to stop spinning. Removing the Flask and Clean-Up 1. Turn off the vacuum line, and carefully release the stopper so that the vacuum seal is broken. 2. Remove the clip, and unhook the flask containing your solid, liquid, or oil sample in the same way that you connected it during Step 4 of the preparation procedure. At this point, you will have obtained your desired material, and have completed the rotary evaporation. Store your sample carefully, and proceed to clean-up. a. As before, do not let go of the flask as it will fall off once you remove the clip. b. You may wish to pull a hard vacuum on the sample for 5-10 minutes to ensure all residual solvent is removed. 3. Deactivate the water flow and heat if you are no longer operating the device. 4. Unclamp the bottom waste reservoir (G), and dispose of the contents in your laboratory’s waste disposal bin. a. The clamp may be difficult to remove, or made of sharp metal, so take care with it. b. Caution: As before, be careful with the reservoir as it can spill. 5. Reattach the waste reservoir, and ensure that it is attached. 6. You have now completed every task necessary to operate and maintain a rotary evaporator at a basic level. Enjoy having to perform this entire process again when your sample is found to be impure or incorrect.