Velindre NHS Trust Commentary –April 2015
advertisement

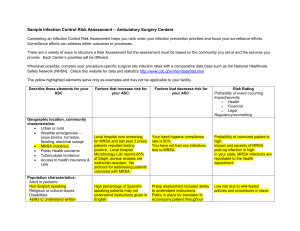
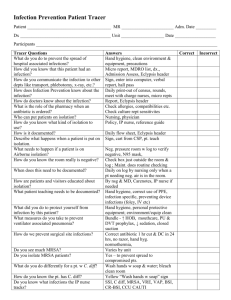
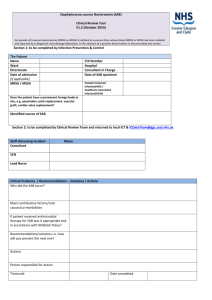
Velindre NHS Trust Commentary –April 2015 Background Velindre NHS Trust takes its commitment to prevent and reduce the risks of a patient picking up an infection whilst being treated in this hospital extremely seriously. We know that our patients are particularly vulnerable to infection either as a result of the cancer weakening their immune system or from treatments such as chemotherapy and radiotherapy that actively reduce their ability to fight infections. Velindre Cancer Centre is a specialty cancer (oncology) hospital with up to 50 inpatient beds. It provides non-surgical specialist services to a population of 1.5 million across South East Wales and so treats many more outpatients and day cases than in-patients. Due to the specialist services and small number of in-patient beds, our infection numbers are not directly comparable with Health Boards in Wales. Therefore the rate of infection for Velindre is reported per 1000 admissions to the hospital rather than the 100,000 population used to calculate the figures for Health Boards. We carefully monitor our progress and numbers of infections every month, quarterly and annually. We also, where possible, try and compare some of our information with other cancer centres in England. How are we Doing? Overall the information in the accompanying graphs shows that the Trust has made significant reductions in key infections since 2010. Clostridium difficile (C. diff) Diarrhoea C. difficile bacterial spores spread very easily in the clinical environment without good control measures but we have been successful in reducing the number of C.diff infections (in all age groups) in the Cancer Centre over the last 5 years: 8 cases in 2014/15 14 cases in 2013/14 13 cases in 2012/13 21 cases in 2011/12 25 cases in 2010/11 Each infection has been investigated to identify the root cause of the patient infection. We had seen a small increase in the number of cases April 2013 to March 2014 particularly in those patients aged 66yrs and older but each case was thoroughly investigated and in the majority of cases there was no evidence to suggest that the rise was due to cross infection. In four cases where cross infection was a factor and we took steps to reduce the risk further. These steps have led to a 42% reduction for 2014/15 on the previous year with only 1 of these cases identified as potentially hospital acquired. Cases of C. diff are often caused by the use of antibiotics, rather than cross infection and as our patients often require more than one course of antibiotics to prevent and treat serious infections, they are at increased risk of developing C.diff. In spite of this added risk we have been able to successfully reduce the number of C.diff infections over the last 5 years. This has exceeded the reduction target set by the Trust for each year for this infection and over the 5 years a 68% reduction in cases has been achieved. We take all cases of C. diff. very seriously and continue to take steps to: increase awareness in staff, promote the need for vigilance in infection control precautions and hand hygiene, review all antibiotics prescribed and enhance the cleaning of the clinical area with specific disinfectants. The Infection Control Doctor undertakes ward rounds with the antimicrobial pharmacist on a weekly basis where possible. Further case reviews have been undertaken and we are also ensuring effective communication with clinical staff so that any ‘lessons learnt’ can be shared, to ensure the delivery of safe and effective treatment and care. Meticillin Resistant Staphylococcus aureus (MRSA) Blood Stream Infections (bacteraemia) Due to the nature of the care provided at Velindre Cancer Centre, bloodstream infection or bacteraemia is a recognised risk especially when a central intravenous device is required to deliver chemotherapy or if the patient is already carrying MRSA on their skin. It can be seen that we have a very low rate of MRSA blood stream infection: 0 cases in 2014/15 2 cases in 2013/14. 0 cases in 2012/13 1 case in 2011/12 4 cases in 2010/11 Each of these infections has been investigated and only 1 identified in 2010 was recorded as acquired in this hospital. We have identified that in all cases the MRSA bacteria was already being carried on the patient’s skin before they were admitted to Velindre and before the blood stream infection occurred. We actively screen all patients for MRSA skin carriage. Compliance with the MRSA policy on the wards is audited monthly to ensure patients are screened: on their first admission if admitted from another hospital if they have had MRSA previously prior to surgical procedures prior to the insertion of central venous devices. This policy allows us to try and reduce MRSA on the patient’s skin (if they are found to be carrying the bacteria) and reduce risks of others being exposed to the bacteria in the clinical environment. Meticillin Sensitive Staphylococcus aureus (MSSA) Blood Stream Infections (bacteraemia) Staphylococcus aureus is a common skin bacteria/organism that can cause a bloodstream infection. MSSA is commonly associated with the use of intravenous devices and may be due to contamination from the patient’s own skin bacteria. Numbers of MSSA bloodstream infections in Wales have increased by 12% and similar rises have been seen at Velindre but we also have very low rate of MSSA blood stream infection: 2 cases in 2014/15 7 cases in 2013/14 7 cases in 2012/13 4 cases in 2011/12 Since 2011 we have only identified three cases of MSSA infection where the infection might have been acquired at the Cancer Centre. Though the numbers of infections for Velindre are small, each MSSA bacteraemia has been investigated. We do know that the majority of these infections occurred in patients with central intravenous devices. These devices are known as PICC or Hickman lines. They pierce the skin when inserted and may need to stay in place for up to 18 months to deliver chemotherapy. These infections may not be attributed to cross infection or infection introduced during their hospital stay. Velindre NHS Trust is committed to trying to reduce the number of MSSA infections in patients. Between April 2013 and March 2014 there have been only 2 cases of MSSA bacteraemia and neither of these were Velindre acquired. This is 4 less cases than the same time period in the previous year. We take all blood stream infections very seriously and have an active programme for reviewing each case and auditing clinical practices for intravenous device insertion and maintenance. Goal’s/Targets Velindre NHS Trust takes a ‘zero’ tolerance approach to healthcare associated infection and strives to eliminate all preventable HCAIs. We are using the 1000 lives plus improvement methods and using national guidance to guide our practice including the National standards of Cleanliness and WHO hand hygiene programme (http://www.1000livesplus.wales.nhs.uk/hcai). Our objectives include: To prevent an increase in the number of C diff. infections for the year 2015/16 focusing on all age groups but especially those patients aged over 66yrs of age. This is while accepting that, due to the small numbers involved, further reductions may be difficult to achieve To continue to review antibiotic prescribing and ensure our antibiotic policy minimises, where possible, unnecessary use of antibiotics especially those that increase the risk of C. diff. infection. To implement the Velindre action plan for antimicrobial prescribing in response to the recent nationally published antimicrobial prescribing report. To continue to comply with best practice for the care of central intravenous devices and investigate all MRSA and MSSA blood stream infections and explore new interventions to reduce the risk further including new technologies. To continue to use the 1000 Lives plus methods for improvement of the care of medical devices e.g. urinary catheters and peripheral intravenous devices that are often associated with bloodstream infection. To actively screen all new patients admitted to Velindre for MRSA especially those who require a central intravenous device or invasive procedure so we can take precautions for those who are carrying it already on their skin and in their nose. To monitor compliance with the National Standards of Cleanliness to ensure our environment is clean and safe where care is provided. To continue to monitor monthly compliance with good hand hygiene practices in all patient areas. To audit clinical practices and use improvement methodologies to improve patient care and reduce the risks of healthcare associated infections