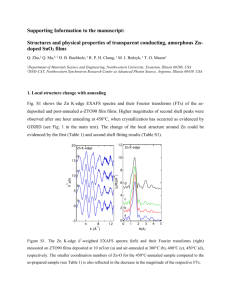
View/Open

SUPPLEMENTARY MATERIAL
Iron colloids reduce the bioavailability of phosphorus to the green alga Raphidocelis subcapitata
S
TIJN
B
AKEN
*, S
OPHIE
N
AWARA
, C
HRISTOFF
V
AN
M
OORLEGHEM AND
E
RIK
S
MOLDERS
KU Leuven, Department of Earth and Environmental Sciences, Kasteelpark Arenberg 20 bus
2459, 3001 Leuven, Belgium
* Corresponding author, tel. +3216321761, e-mail: stijn.baken@ees.kuleuven.be
1
Culture conditions
A pure culture of Ralstonia subcapitata was obtained from the Culture Collection of Algae and Protozoa (CCAP 278/4, Oban, U.K.). A subculture was initiated in a cellulose-stoppered
Erlenmeyer flask containing sterile culture medium (a modified WC medium; composition:
Table 1 in the manuscript) which was inoculated with between 2 x 10
3
and 5 x 10
3
cells mL
-1
.
The culture was kept at ambient temperature on an unshaken light cabinet with continuous illumination from the bottom by cool white lamps (Master TL-E Circular Super 80 32W/840,
Philips), and was replaced every 1-2 weeks. A sample of the culture was regularly checked by microscopy and no visual contamination by bacteria or other micro-organisms was observed.
The cell density was monitored by particle counting (HIAC Royco 9705). The exponential growth rate between day 1 and day 4 ranged between 1.0 and 1.1 day -1 .
Algae for the uptake experiments were harvested under sterile conditions from cultures grown for 6—10 days (cell density between 1 x 10
6
and 4 x 10
6
cells mL
-1
). The algae were centrifuged (15 min, 6000 g ), the supernatant was decanted, the algal pellet was washed with ultrapure water, the algae were centrifuged again, the supernatant was decanted again, and the algal pellet was transferred to a sterile P-free medium (composition: Table 1 in the manuscript). The cell density at this stage was around 1 x 10
6
cells mL
-1
. After installation on the unshaken light cabinet, the algae were starved for P for 7—12 days, during which the cell density increased to approximately 3 x 10 6 cells mL -1 . On the day of the uptake experiment, the P-starved algae were isolated and washed in a similar way as described above. The algal pellet was then suspended in an aliquot of water at a cell density of approximately
1 x 10
7
cells mL
-1
for use in the P uptake experiments. The internal P concentration of the algae in this suspension was between 0.4 and 0.6% P on a dry-weight basis compared to around 2% P for cultures grown under adequate P supply. This shows that the culture was indeed P-starved.
2
EXAFS spectroscopy
Freeze-dried subsamples of the colloidal samples A and B were measured by Fe-edge
(7112 eV) EXAFS spectroscopy at the wiggler beamline I811, MAX-lab, Lund, Sweden.
Beamline I811 was equipped with a Si[111] double crystal monochromator. Higher-order harmonics were reduced by detuning the second monochromator crystal to reflect approximately 40% of the maximum intensity at the end of the scans. More details on beamline I811 are provided by Carlson et al. (2006). The samples were mounted in aluminium holders and fixed with Kapton tape. The spectrum of a metallic iron foil was used for energy calibration. The scans were recorded at ambient temperature in fluorescence mode with a Mn filter. For each sample, 11 scans were recorded. Data processing and modelling was performed using the Athena and Artemis programs (Ravel and Newville, 2005). Energy calibration was performed by assigning the first inflection point of the metallic Fe foil spectrum to 7112 eV. All scans of a sample were aligned, averaged, and the background was removed using the Autobk algorithm using a k -weight of 2 and a first-shell fit of the EXAFS spectrum as standard. Standard spectra included those of 2-line ferrihydrite, Fe complexed by
SRNOM, goethite, lepidocrocite, and the Fe(III)-trisoxalate complex. For the preparation of the Fe-SRNOM complex, a solution containing 10 mg SRNOM L
-1
, 0.9 µmol Fe(II) L
-1
(added as FeSO
4
·7H
2
O), and 2 mM NaHCO
3
buffer at pH 7.2 was oxidised for 24 hours and then freeze-dried. This resulted in a standard containing 90 µmol added Fe (g SRNOM) -1 . The goethite, lepidocrocite, and Fe(III)-trisoxalate spectra were obtained from earlier studies
(Kleja et al., 2012; Sjöstedt et al., 2013; van Schaik et al., 2008).
The resulting k
3
-weighted EXAFS spectra were analysed between k = 2.6 and 12.0 Å
-1
by the methods of wavelet transforms (WT), linear combination fitting (LCF), and conventional
EXAFS modelling. The WT of EXAFS spectra can be used to qualitatively discriminate between the nature of the backscattering atoms. Heavy backscattering atoms (such as Fe)
3
cause oscillations at high k values, whereas lighter backscattering atoms cause oscillations at lower k values. We use the WT method in order to detect the presence of Fe backscattering atoms in the second and third coordination shells, which causes a pronounced maximum in the WT plot at around k = 7 Å -1 and R = 3 Å (Karlsson et al., 2008). Plots of the WT modulus were calculated using the method developed by Funke et al. (2005) (parameters: η = 8, σ = 1) which is implemented in the Igor Pro script. Linear combination fitting (LCF) was applied to the k
3
-weighted EXAFS spectra using a least-squares algorithm. The sum of the contributions of all standards was constrained to equal 1. In order to address the uncertainty associated with
LCF due to noise at high k-values, the higher limit of the k -range was varied between 8.5 and
12.0 Å
-1
. The EXAFS spectra of the colloidal samples and of ferrihydrite were modelled in
Artemis after Fourier-transformation between 2.6 and 12 Å
-1
using a Hanning window. An
EXAFS model similar to the ones used in Mikutta (2011) and Baken et al. (2013) was fitted to the Fourier transformed EXAFS spectra between 1 and 4 Å, and fits were optimised across k weights of 1, 2, and 3. The model included single scattering Fe-O and multiple scattering Fe-
O-O interactions in the first coordination shell, a single scattering Fe-Fe2 interactions in the second coordination shell, and a single scattering Fe-Fe3 interaction in the third coordination shell. This model contained a total of 14 parameters, 7 of which were fixed or constrained
(
Ошибка! Источник ссылки не найден.
).
4
Figure SM1: Schematic of the experimental set-up.
5
Colloids A
9000 µmol Fe (g SRNOM)
-1
4.0
4.0
Colloids B
900 µmol Fe (g SRNOM)
-1
3.0
3.0
2.0
2 6 10
4.0
Fe-SRNOM complex
2.0
2 6
Ferrihydrite
10
4.0
3.0
3.0
2.0
2 6
k (Å
-1
)
10
2.0
2 6
k (Å
-1
)
10
Figure SM2: Wavelet transform modulus of the EXAFS spectra of colloidal samples and standards. The maximum around k = 7 Å -1 and R = 2.8 Å refers to Fe-Fe interactions.
6
0.30
0.20
0.10
0.00
0
0.20
0.10
Treatment 1 y = 0.0034x + 0.0558
R² = 0.9924
0.30
0.20
Treatment 2 y = 0.0046x + 0.0286
R² = 0.9749
0.30
Treatment 3 y = 0.0036x + 0.0196
R² = 0.9955
0.20
0.10
20 40
0.10
60
0.00
0 20 40 60
0.00
0 20 40
Treatment 4 Treatment 5 Treatment 6 y = 0.0036x + 0.026
R² = 0.9948
0.20
y = 0.0027x + 0.0134
R² = 0.9908
0.20
y = 0.0026x + 0.0131
R² = 0.9901
0.10
0.10
60
0.00
0
0.10
0.05
0.00
0
0.20
0.15
0.10
0.05
0.00
0
0.02
20 40 60
0.00
0 20 40 60
0.00
0 20 40 60
Treatment 7 Treatment 8 Treatment 9
0.10
0.15
y = 0.0012x + 0.0079
R² = 0.9531
y = 0.0016x + 0.0151
R² = 0.9891
0.10
y = 0.0022x + 0.0065
R² = 0.9386
0.05
0.05
20 40 60
0.00
0 20 40 60
0.00
0 20 40 60
Treatment 10 Treatment 11 Treatment 12
0.20
0.20
y = 0.0026x + 0.0246
y = 0.0021x + 0.0244
R² = 0.9837
0.15
R² = 0.9923
0.15
y = 0.0023x + 0.0201
R² = 0.9615
0.10
0.10
0.05
0.05
20 40 60
0.00
0 20 40 60
0.00
0 20 40 60
Treatment 13 Treatment 14 y = 0.0001x + 0.0087
R² = 0.6977
0.01
y = 0.0002x + 2E-05
R² = 0.9009
0.01
0.00
0 20 40 60
0.00
0 20 40 60
Figure SM3: Plots of internalised
33
P fraction (y-axis; dimensionless) versus time (x-axis; in minutes) for each treatment. The internalised
33
P fraction was calculated as the measured
33
P activity in the algal pellet divided by the total
33
P activity. The full line is the fitted linear regression model.
7
References
Baken, S., Sjöstedt, C., Gustafsson, J.P., Seuntjens, P., Desmet, N., De Schutter, J., Smolders,
E., 2013. Characterisation of hydrous ferric oxides derived from iron-rich groundwaters and their contribution to the suspended sediment of streams. Appl. Geochemistry 39, 59–
68.
Carlson, S., Clausén, M., Gridneva, L., Sommarin, B., Svensson, C., 2006. XAFS experiments at beamline I811, MAX-lab synchrotron source, Sweden. J. Synchrotron
Radiat. 13, 359–364.
Funke, H., Scheinost, A., Chukalina, M., 2005. Wavelet analysis of extended x-ray absorption fine structure data. Phys. Rev. B 71, 1–7.
Karlsson, T., Persson, P., Skyllberg, U., Mörth, C.-M., Giesler, R., 2008. Characterization of iron(III) in organic soils using extended X-ray absorption fine structure spectroscopy.
Environ. Sci. Technol. 42, 5449–54.
Kleja, D.B., van Schaik, J.W.J., Persson, I., Gustafsson, J.P., 2012. Characterization of iron in floating surface films of some natural waters using EXAFS. Chem. Geol. 326-327, 19–
26.
Mikutta, C., 2011. X-ray absorption spectroscopy study on the effect of hydroxybenzoic acids on the formation and structure of ferrihydrite. Geochim. Cosmochim. Acta 75, 5122–
5139.
Ravel, B., Newville, M., 2005. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for Xray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–41.
Sjöstedt, C., Persson, I., Hesterberg, D., Kleja, D.B., Borg, H., Gustafsson, J.P., 2013. Iron speciation in soft-water lakes and soils as determined by EXAFS spectroscopy and geochemical modelling. Geochim. Cosmochim. Acta 105, 172–186.
Van Schaik, J.W.J., Persson, I., Kleja, D.B., Gustafsson, J.P., 2008. EXAFS study on the reactions between iron and fulvic acid in acid aqueous solutions. Environ. Sci. Technol.
42, 2367–73.
8