Pharmacology 20 [5-11
advertisement

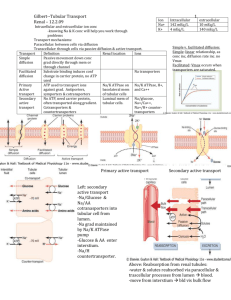
Pharmacology 20: Volume Regulation - - - - - - Fluid in vascular compartment determines extent of tissue perfusion 2/3 of body water = intracellular and 1/3 = extracellular (3/4 in interstitial space, ¼ in plasma) Between plasma and interstitial compartments, capillary permeability (jxns between cells), oncotic pressure (molecular solute components) and hydrostatic pressure allows exchange o Fluid filtration = Kf (Pc – Pif) – (πc – πif) o Hydrostatic and oncotic gradient terms have opposing vectors o At arterial end of capillary bed, plasma filtration favored. At venous end, balanced or favors flow from interstitium to capillary bed. Liver returns fluids via lymphatic flow Low-pressure vascular volume sensors = atria and pulmonary vasculature o Low wall stress signals PNS to transmit signal to noradrenergic neurons in medulla -> hypothalamus -> increase ADH/vasopressin secretion o Increased wall stress, atria secreted natriuretic peptide -> vasodilation and natriuresis High-pressure vascular volume sensors = baroreceptors in aortic arch, carotid sinus, and JGA apparatus o Modulate hypothalamic control of ADH secretion and sympathetic outflow, renin Neurohormonal response (volume regulators) = renin-angiotensin-aldosterone system (RAAS), natriuretic peptides, ADH, and renal sympathetic nerves Renin produced and secreted by juxtaglomerular apparatus (JGA) -> vasoconstriction and Na+ retention, maintaining tissue perfusion and increase ECF volume o Direct pressure-sensing mechanism of afferent arteriole increases renin with decrease wall tension o Sympathetic innervation of JGA cells promotes renin via β1-adrenoreceptor signal o Tubuloglomerular feedback senses distal nephron Cl or Na delivery modulating renin distal end of thick ascending limb apposed to JGA (rapid regulation) Macula densa of thick ascending limb respond to increased NaCl delivery (increase extracellular adenosine activating A1 receptors on JGA to decrease renin). Decreased NaCl -> prostaglandin signaling cascade with renin release NaCl concentration senses by receptors in monocilia of macula densa. Luminal fluid flow rate sensed by direct bend of monocilia. Renin cleaves angiotensinogen to generate angiotensin I cleaved by angiotensin converting enzyme (ACE I) to active angiotensin II (AT II) o ACE I in pulmonary vascular endothelium and coronary circulation but regulates ATII production in all vascular beds o ACE is aka as kininase II (broad proteolytic specificity including kinins) ATII binds to AT II receptor subtype I (AT1R) -> stimulates aldosterone secretion (zona glomerulosa cells in adrenal glands), increased NaCl reabsorption in proximal tubule, stimulation of thirst and ADH, arteriolar vasoconstriction o Aldosterone increases distal-tubule Na reabsorption o - - - - - - AT1R (G-protein) activates phospholipase C -> Ca release (increase vascular smooth muscle contractility, vascular resistance) AT2R is more prominent in fetal than adult tissue (vasodilatory role) Natriuretic peptides (atria, ventricles and vascular endothelium) released w/ overload o A-type (ANP) = primarily by atria o B-type (BNP) = primarily by ventricles o C-type (CNP) = by vascular endothelial cells o Uroguanylin (UGN) = enterocytes in response to dietary salt o Vascular NPs released in response to increased intravascular volume (stretch of cells) Bind to NPR-A, NPR-B, and NPR-C NPR-A & B -> transmembrane protein with cytoplasmic guanylyl cyclase (increase cGMP). ANP and BNP bind to NPR-A; CNP binds to NPR-B. NPR-C -> no guanylyl cyclase; decoy or bugger to reduce NPs. Binds all 3 NPs UGN bins transmembrane guanylyl cyclase C in renal proximal tubule cells and enterocytes, undefined receptor in renal collecting ducts NPs affect cardiovascular, kidney, and CNS o relaxes vascular smooth muscle -> dephos of myosin light chain via cGMP; increases capillary permeability o promotes ↑ GFR (efferent arteriole constriction, dilation of afferent) and naturesis (antagonism of ADH) o decreased thirst, ADH and sympathetic tone (via CNP expressed high in brain) ADH or vasopressin is nonpeptide hormone from posterior pituitary from increased plasma osmolality or hypovolemia -> constricts vasculature and ↑ H2O reabsorption o V1 receptor -> in smooth muscle cells; vasoconstriction (Gq) o V2 receptor -> in collecting duct cells; H2O reabsorption (Gs -> cAMP -> PKA -> phosphorylated aquaporin 2 -> porin fusion in apical membrane) Regulation of renal H2O reabsorption in collecting duct modulates urine and plasma osmolality Renal sympathetic nerves in afferent and efferent arterioles -> decrease GFR (with low volume) with constriction of afferent > efferent arteriole -> decreased natriuresis o Increase renin by β1 adrenergic receptors in JGA To alter body fluid volume, alter renal Na reabsorption o NaCl reabsorption is key for water retention Glomerulus produces ultrafiltrate of plasma processed by nephron (solute and H2O reabsorption, waste product and exnobiotic excretion) o Substrate-specific transporters and channels in luminal tubular epithelial cells alter [solute] altered by channels and transporters on contraluminal side Segments of nephron: proximal tubule, thick ascending limb (TAL) of loop of henle, distal convoluted tubule (DCT), and cortical collecting duct (CCD) o Transport of solute and water through transcellular transporters, of ions via paracellular tight junctions - - - - Proximal tube (PT) is first reabsorptive site (2/3 of Na reabsorption, 90% HCO3 reabsorption, 60% of Cl reabsorption). o Symporters drive reabsorption of all glucose, AAs, phosphate and sulfate o Secretion and reabsorption of weak acids/bases (symport or antiport) o HCO3 reabsorption: 2/3 of proton efflux exchanged for Na influx via NHE3 Na/H exchanger and 1/3 by vacuolar H-ATPase. Luminal membrane has glycosylphosphatidylinositol-linked exoenzyme carbonic anhydrase IV (CAIV) -> converts HCO3 to CO2 and OH (enter cell) CO2 rehydratede to HCO3 via carbonic anhydrase II (CAII) -> cotransported with Na across membrane of epithelial cell (Na/HCO3 co-transporter NBCe1 -> 3 HCO3 for 1 Na) o Iso-osmotic solute absorption (H2O accompanies to maintain osmotic balance) Aquaporins (AQP1) -> transcellular water flow in proximal and thin descending limb of loop of Henle Thick ascending limb (TAL) + distal convoluted tubule (DCT) + connecting tubule (CNT) = “diluting segment” TAL receives hypertonic fluid with high NaCl concentration o Devoid of aquaporins (reabsorb NaCl and urea without water) -> solute that generates corticomedullary osmotic gradient of kidney (“countercurrent multiplier”) o Reabsorbs 25-35% of filtered Na by luminal Na/K/2Cl cotransporter NKCC2 Cl exits via CLC-K2 channel and Na via Na/K ATPase ((both on basolateral side) Apical ROMK recyvles K back into lumen Generate lumen-positive electrical potential for paracellular ion absorption o 50% Na reabsorption via paracellular path (allows Na to flow without E waste) o Ca and Mg reabsorbed via cation-channels (paracellins or claudin) in tight jxns DCT reabsorbs 2-10% of NaCl filtered o Luminal Na enters via NCC Na/Cl cotransporter and exits via Na/K ATPase and electrogenic Cl channels and electroneutral K/Cl cotransport o Ca and Mg reabsorbed via TRPV5 (Ca) and TRPM6 (Mg) in apical membrane Ca exits via NCX Na/Ca exchanger and Ca ATPase Terminal nephron = cortical, outer medullary and inner medullary collecting duct (CD). o Cortical and outer medullary CD cells Principal cells -> reabsorb 1-5% of filtered Na depending on aldosterone (Na enters via Na channels (ENaC) and exit via Na/K ATPase). Secrete K into lumen, have ADH-responsive water channels (Gs V2 receptor inserts AQP2) intercalated cells -> express H-ATPase, mediate K absorption by luminal H/K ATPase and NH4 secretion by Rh antigen proteins Type A IC secrete protons via apical H-ATPase, reabsorb HCO3 via basolateral Cl/HCO3 exchanger (kidney AE1) Type B IC secrete HCO3 via apical Cl/HCO3 exchanger pendrin and reabsorb protons via basolateral H-ATPase - - - - - NonA, nonB IC reabsorbs CL via pendrin Edema = accumulation of fluid in interstitial space o Exudative (high protein; inflamm response) or transudative (low protein; pathologic renal Na retention) Return to physiologic homeostasis mediated by: osmotic force, lumphatic drainage, and longterm volume sensors o Pathophysiology of transudative edema formation almost always requires an element of pathologic renal Na retention o Heart failure, cirrhosis and nephrotic syndrome -> all deranged Na reabsorption Heart failure (HF) = inability of heart to perfuse tissues/organs -> congestion in venous “capacitance” vessels *increase capillary hydrostatic pressure) o RHF -> peripheral edema; LHF -> pulmonary edema o Cause of Na retention is perceived volume depletion (high-pressure volume receptors) -> increased renin-angiotensin-aldosterone system (endothelin and prostaglandins, too) Low-pressure NP and neural response sense increased pressure promoting natriuresis but disrupted in HF (too much sympathetic response) o Diuretics and ACE inhibitors can be used Cirrhosis = hepatic parenchymal fibrosis from chronic inflammation or hepatotoxic insult o Portosystemic shunt from liver into systemic circulation; decreased albumin, contributors to plasma oncotic pressure, and coagulation factors o Underfill model -> obstruction of venous outflow increases intrahepatic hydrostatic pressure increasing transudation overwhelming lymphatic flow -> ascites ↓ intravascular volume, Q, and activation of baroreceptors (↑ Na retention) o Overflow model -> ascites from primary renal Na retention. Obstruction activates hepato-renal reflex (↑ Na retention) Nephrotic Syndrome = massive proteinuria, edema, hypoalbuminemia, hypercholesterolemia o Primary cause = glomerular dysfunction o Massive proteinuria -> ↓ plasma oncotic pressure ->fluid transudation into interstitium -> ↓ intravascular volume -> volume sensors enhance Na retention o May be caused by change in capillary jxnal permeability or primary Na retention (localized to distal nephron) o Treat with diuretics but correct glomerular disorder