Polysaccharides
advertisement

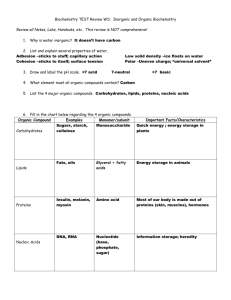
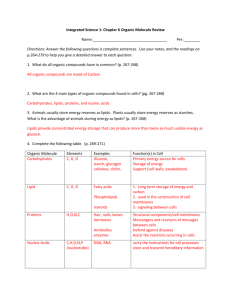
Organic Compounds Lesson Objectives Explain why carbon is essential to life on Earth. Describe the structure and function of carbohydrates. Describe the structure and function of lipids. Describe the structure and function of proteins. Describe the structure and function of nucleic acids. Introduction Organic compounds are chemical substances that make up organisms and carry out life processes. All organic compounds contain the elements carbon and hydrogen. Because carbon is the major element in organic compounds, it is essential to all known life on Earth. Without carbon, life as we know it could not exist. The Significance of Carbon Why is carbon so important to organisms? The answer lies with carbon’s unique properties. Carbon has an exceptional ability to bind with a wide variety of other elements. Carbon atoms can form multiple stable bonds with other small atoms, including hydrogen, oxygen, and nitrogen. Carbon atoms can also form stable bonds with other carbon atoms. In fact, a carbon atom may form single, double, or even triple bonds with other carbon atoms. This allows carbon atoms to form a tremendous variety of very large and complex molecules. Nearly 10 million carbon-containing organic compounds are known. Types of carbon compounds in organisms include carbohydrates, lipids, proteins, and nucleic acids. The elements found in each type are listed in Table 1. Elements other than carbon and hydrogen usually occur within organic compounds in smaller groups of elements called functional groups. When organic compounds react with other compounds, generally just the functional groups are involved. Therefore, functional groups generally determine the nature and functions of organic compounds. Type of Compound Carbohydrates Lipids Proteins Organic Compounds Elements it Contains Carbon, hydrogen, oxygen Carbon, hydrogen, oxygen Examples Glucose, Starch, Glycogen Cholesterol, Triglycerides (fats) Phospholipids Enzymes, Antibodies Carbon, hydrogen, oxygen, nitrogen, sulfur Nucleic Acids Carbon, hydrogen, oxygen, Deoxyribonucleic acid (DNA) nitrogen, phosphorus Ribonucleic acid (RNA) This table lists the four types of organic compounds, the elements they contain, and examples of each type of compound Organic Compounds Carbohydrates Carbohydrates are organic compounds that contain only carbon, hydrogen, and oxygen. They are the most common of the four major types of organic compounds. There are thousands of different carbohydrates, but they all consist of one or more smaller units called monosaccharides. The general formula for a monosaccharide is: (CH2O)n, where n can be any number greater than two. For example, if n is 6, then the formula can be written: C6H12O6. This is the formula for the monosaccharide glucose. If two monosaccharides bond together, they form a carbohydrate called a disaccharide. An example of a disaccharide is sucrose (table sugar), which consists of the monosaccharides glucose and fructose. Polysaccharides If more than two monosaccharides bond together, they form a carbohydrate called a polysaccharide. A polysaccharide may contain anywhere from a few monosaccharides to several thousand monosaccharides. Polysaccharides are also called complex carbohydrates. Their main functions are to store energy and form structural tissues. Lipids Lipids are organic compounds that contain mainly carbon, hydrogen, and oxygen. They include substances such as fats and oils. Lipid molecules consist of fatty acids, with or without additional molecules. Fatty acids are organic compounds that have the general formula CH3(CH2)nCOOH. Lipids and Diet Humans need lipids for many vital functions, such as storing energy and forming cell membranes. Lipids can also supply cells with energy. In fact, a gram of lipids supply more than twice as much energy as a gram of carbohydrates or proteins. Lipids are necessary in the diet for most of these functions. Although the human body can manufacture most of the lipids it needs, there are others, called essential fatty acids, that must be consumed in food. Essential fatty acids include omega-3 and omega-6 fatty acids. Both of these fatty acids are needed for important biological processes, not just for energy. Although some lipids in the diet are essential, excess dietary lipids can be harmful. Because lipids are very high in energy, eating too many may lead to unhealthy weight gain. A high-fat diet may also increase lipid levels in the blood. This, in turn, can increase the risk for health problems such as cardiovascular disease. The dietary lipids of most concern are saturated fatty acids, trans fats, and cholesterol. For example, cholesterol is the lipid mainly responsible for narrowing arteries and causing the disease atherosclerosis. Organic Compounds Proteins Proteins are organic compounds that contain carbon, hydrogen, oxygen, nitrogen, and, in some cases, sulfur. Proteins are made of smaller units called amino acids. There are 20 different common amino acids needed to make proteins. All amino acids have the same basic structure. Only the side chain differs from one amino acid to another. The variable side chain gives each amino acid unique properties. Proteins can differ from one another in the number and sequence (order) of amino acids. It is because of the side chains of the amino acids that proteins with different amino acid sequences have different shapes and different chemical properties. Functions of Proteins Proteins are an essential part of all organisms. They play many roles in living things. Certain proteins provide a scaffolding that maintains the shape of cells. Proteins also make up the majority of muscle tissues. Many proteins are enzymes that speed up chemical reactions in cells; other proteins are antibodies. Antibodies bond to foreign substances in the body and target them for destruction. Still other proteins help carry messages or materials in and out of cells or around the body. For example, the blood protein hemoglobin bonds with oxygen and carries it from the lungs to cells throughout the body. Proteins and Diet Proteins in the diet are necessary for life. Dietary proteins are broken down into their component amino acids when food is digested. Cells can then use the components to build new proteins. Humans are able to synthesize all but eight of the twenty common amino acids. These eight amino acids, called essential amino acids, must be consumed in foods. Like dietary carbohydrates and lipids, dietary proteins can also be broken down to provide cells with energy. Nucleic Acids Nucleic acids are organic compounds that contain carbon, hydrogen, oxygen, nitrogen, and phosphorus. They are made of smaller units called nucleotides. Nucleic acids are named for the nucleus of the cell, where some of them are found. Nucleic acids are found not only in all living cells but also in viruses. Types of nucleic acids include deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is the genetic information of the cell which tells the cell when to grow, by how much, and controls all other functions of the cell. Organic Compounds Lesson Summary Carbon’s exceptional ability to form bonds with other elements and with itself allows it to form a huge number of large, complex molecules called organic molecules. These molecules make up organisms and carry out life processes. Carbohydrates are organic molecules that consist of carbon, hydrogen, and oxygen. They are made up of repeating units called saccharides. They provide cells with energy, store energy, and form structural tissues. Lipids are organic compounds that consist of carbon, hydrogen, and oxygen. They are made up of fatty acids and other compounds. They provide cells with energy, store energy, and help form cell membranes. Proteins are organic compounds that consist of carbon, hydrogen, oxygen, nitrogen, and, in some cases, sulfur. They are made up of repeating units called amino acids. They provide cells with energy, form tissues, speed up chemical reactions throughout the body, and perform many other cellular functions. Nucleic acids are organic compounds that consist of carbon, hydrogen, oxygen, nitrogen, and phosphorus. They are made up of repeating units called nucleotides. They contain genetic instructions for proteins, help synthesize proteins, and pass genetic instructions on to daughter cells and offspring. Review Questions 1. What elements must be present to make an organic compound? 2. State the difference between a monosaccharide and polysaccharide. 3. Fats and oils are examples of what? 4. Of lipids, carbohydrates and proteins, which one supplies the most energy? 5. Nucleotides come together to form what? 6. Name the two main types of nucleic acids. 7. Why is carbon essential to all known life on Earth? 8. Compare and contrast simple sugars and complex carbohydrates. 9. Why are some amino acids known to be essential? 10. Provide examples of carbohydrates, lipids, proteins, and nucleic acids. Organic Compounds A. Vocabulary 1. amino acid= Small organic molecule that is a building block of proteins. 2. Carbohydrate= Type of organic compound that consists of one or more smaller units called monosaccharides. 3. Cholesterol= Type of steroid that is an important part of cell membranes and plays other vital roles. 4. complementary bases= Nucleic acid bases that form bonds with each other and help hold together two nucleotide chains. 5. complex carbohydrate= Another term for a polysaccharide. 6. deoxyribonucleic acid (DNA)= Double-stranded nucleic acid that contains the genetic instructions for proteins. 7. Disaccharide= Small carbohydrate, such as sucrose, that consists of two monosaccharides. 8. double helix= Normal shape of a DNA molecule in which two chains of nucleotides are intertwined. 9. essential amino acids= Amino acids that the human body needs but cannot make and must consume in food. 10. essential fatty acids= Fatty acids that the human body needs but cannot make and must consume in food. 11. fatty acid= Organic compound found in lipids that has the general formula CH3(CH2)nCOOH. 12. functional group= Small group of elements within an organic compound that determines the nature and function of the organic compound. 13. Lipid= Type of organic compound that consists of one or more fatty acids with or without additional molecules. 14. Monosaccharide= Small carbohydrate, such as glucose, with the general formula (CH2O)n. 15. nucleic acid= Type of organic compound that consists of smaller units called nucleotides. 16. Nucleotide= Small organic molecule that is a building block of nucleic acids. 17. Peptide= Short chain of amino acids. 18. Phospholipid= Type of lipid that is a major component of cell membranes. 19. Polypeptide= Long chain of amino acids. 20. Polysaccharide= Large carbohydrate that consists of more than two monosaccharides. 21. Protein= Type of organic compound that consists of smaller units called amino acids. Organic Compounds 22. ribonucleic acid (RNA)= Single-stranded nucleic acid that uses information contained in DNA to assemble amino acids and make proteins. 23. saturated fatty acid= Type of fatty acid in which all the carbon atoms are bonded to as many hydrogen atoms as possible. 24. simple sugar= Another term for a monosaccharide or disaccharide. 25. Steroid= Type of lipid that has several functions, such as forming cell membranes and acting as sex hormones. 26. trans fatty acid= Artificial, unsaturated fatty acid that has properties similar to saturated fatty acids. 27. Triglyceride= Type of lipid that is the main form of stored energy in animals. 28. unsaturated fatty acid= Type of fatty acid in which some carbon atoms are not bonded to as many hydrogen atoms as possible. Reference: Benson, Alan. (n.d.). Section 2.2: Organic Compounds. cK-12 website. Retrieved from http://www.ck12.org/user:YXJiZW5zb25AY2RzY2hvb2xzLm9yZw../section/Section2.2%253A-Organic-Compounds/