Supplementary Information: S1. Hydrogen
advertisement

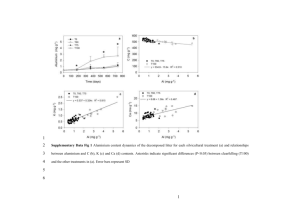
Supplementary Information: S1. Hydrogen-assisted Temperature Programmed Decomposition of Oxalate Precursors The H2-TPDec of the Co1Cu1Mn1 ternary oxalate (Fig. S1) shows a major feature at temperatures between 350 and 390 °C while smaller amounts do so between ~250 °C and the onset of the high-temperature feature. CO and CO2 formation are correlated with H2 Carbon species outlet (a.u.) consumption over the entire range of temperatures. CO2 CO H2 240 280 320 400 360 440 480 o Temperature ( C) Fig. S1 Formation of CO and CO2 along with H2 consumption during H2-TPDec (3°C.min-1) of Co1Cu1Mn1 (solid line) and Co1Cu1Mo1 (dashed line). The decomposition of the hybrid-type Co1Cu1Mo1 precursor shows peaks at 280 °C, 320 °C and 400 °C which correlate with H2 consumption. Further hydrogen consumption occurs beyond the high-temperature decomposition peak at 420 °C, indicating that partial reduction of MoVI takes place in the presence of CoCu. This is, at present, a tentative explanation as the MoVI → MoIV transition (in the absence of CoCu) is known to take place at higher temperatures [1]. 1 Different from the binary CoCu-oxalate, the quantitative evaluation of the TPDec data for the ternary system of both “CoCuMn” and “CoCuMo” demonstrates that oxygen is retained in the catalyst when reaching the high-end of the examined temperature range. Thus, neither Mn-oxalate nor ammonium heptamolybdate were reduced to Mn or Mo metallic states, which is in agreement with what the literature reports [1, 2]. TPDec studies with pure Mn-oxalate confirmed non-stoichiometric MnOx (SBET=525 m2 g-1) formation [2, 3]. Such MnOx accompanying “CoCuMn” did not prevent the activated catalysts from being pyrophoric and caused a drastic increase in the specific surface area. H2-TPDec measurements up to various temperatures between 150 °C and 500 °C, followed by BET, showed that a maximum surface area of SBET~170 m2 g-1 occurred at 350–370 °C for Co1Cu1Mn1. An identical set of measurements led to SBET~185 m2 g-1 for Co1Cu1Mo1 in the same range of temperatures. Co1Cu1, however, showed a maximum of SBET~10 m2 g-1. We also note that heating to temperatures beyond 350–370 °C resulted in a dramatic loss of the surface areas. 2 S2. Atom Probe Tomography Fig. S2 Atom probe tomography results of a single CoCuMn catalyst nanoparticle. The image provided is a section through a 3D tomographic reconstruction so as to reveal the intragranular structure of the nanoparticle. The data is presented in the form of atom maps where Co atoms are depicted as blue spheres, Cu atoms as orange spheres, Mn atoms as green spheres, and O as white spheres. Atom probe and TEM data (S3) reported here were obtained in the group of S. Ringer, Australian Centre for Microscopy & Microanalysis, and School of Aerospace, Mechanical & Mechatronic Engineering, University of Sydney, NSW, 2006, Australia 3 S3. Transmission Electron Microscopy Fig. S3 Evaluation of catalyst grain, or particle size distribution from TEM analysis. Frames indicate where particle sizes were analysed. Particles smaller than 7 nm were also detected and analyzed by HRTEM. Diffraction demonstrated the existence of Mn-oxides. References [1] Lalik E, David WIF, Barnes P, Turner JFC (2001) J Phys Chem B 105: 9153–9156 [2] Iablokov V, Frey K, Geszti O, Kruse N (2010) Catal Lett 134: 210–216 [3] Frey K, Iablokov V, Sáfrán G, Osán J, Sajó I, Szukiewicz R, Chenakin S, Kruse N (2012) J Catal 287: 30–36 4 5