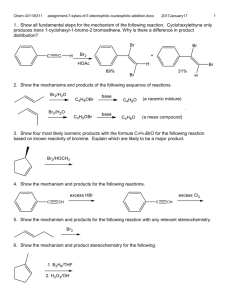
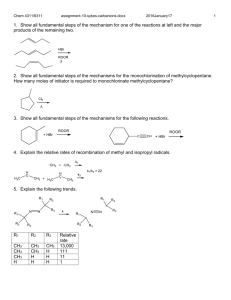
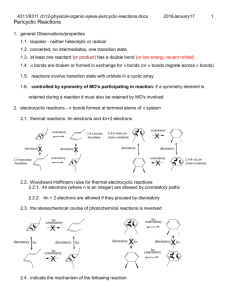
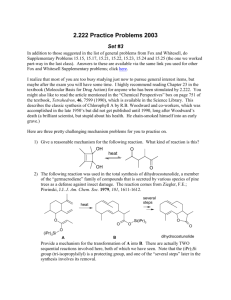
CH14 Free Radical Reactions
advertisement

4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx 2016January17 1 skip 11.3, 11.5.2.1 p326-327 except NBS, 11.5.2.2 p330-331, 11.5.2.3, 11.6 Free Radical Reactions 1. general properties of radicals 1.1. unpaired electron, slightly pyramidal, small energy barrier to invert pyramidal geometry 1.1.1. planar – 5% s character in orbital containing electron 1.1.2. ESR – orbital connecting to electron and 13C – little significant s character 1.1.3. EWG increase s character, why? 1.2. normally very reactive with short lifetimes 1.3. If they don't react with closed shell molecules, they decay by termination (coupling and disproportionation: examples) 1.4. acids, bases and polar solvent have minor affect 1.5. stabilization 1.5.1. alkyl substitution, (methyl < primary < secondary < tertiary) 1.5.1.1. hyperconjugation 1.5.1.2. greater reduction in steric interaction when radical forms 1.5.2. resonance stabilization (allyl, benzyl, heteroatom) – related BDE , 2016January17 4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx 2 1.5.3. steric hindrance inhibit radical combination non planar rings in triphenylmethyl radical 2. Radical formation 2.1. Electron transfer 2.1.1. Chemical reduction A e A O O Na + Na Na R C C R R C C R , H2O2 Ti 3 HO HO Ti 4 O O O + + Cu O O O + O + Cu+2 O 2.1.2. Electrolytic reduction 2.1.3. oxidation: D D e 2.1.3.1. RCO2H Pb4 RCO2 Pb3 H , also works with Ce+4, Mn+3 and Ag+2 2.1.3.2. Electrolytic oxidation: 2.2. thermal cleavage – weak bonds: cumulated lone pairs, stabilized products, organometallics for electronegative metal 4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx 2016January17 O RO OR O 2RO O O 2 O O N N N C C C N N N N C N + N N 2 C N 2.3. photolysis h 2.3.1. cumulated lone pairs: Br2, ROOR 2RO , HOX, 2.3.2. electrons: alkenes and carbonyls 3. Reactions of radicals 3.1. atom abstraction - usually monovalent (H or halogen) 3.1.1. dependent on bond energies, TS can have polar character 3.1.2. R' O RH R' OH R,R alkyl,benzyl , allyl 3.1.3. R3M R' X R3MX R' ,M Sn, Ge & Si, X Cl,Br,I 3.1.4. rates can be estimated where abstraction is rate determining step BDE broken formed CH3CH2-H (98) Br-H (88) CH3CH2-H (98) F-H (136) Ea for Br = 13.2 kcal/mol, H* = 12.6 kcal/mol HOCl HO Cl 3 2016January17 4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx 4 Ea for F = 0.3 kcal/mol, H* = -0.3 kcal/mol 3.1.5. exothermic processes are diffusion controlled, barrier estimated from activation energy (2-5 kcal in solution) endothermic barrier estimated from bond energy difference and reverse barrier (2-5 kcal) 3.2. fragmentation O R O O R + CO2 O R N N R + N O R + C O N R CH3 3.3. Addition 3.3.1. To other radicals: R O2 RO 2 3.3.2. To bonds: R' R2C CR2 R' R2 CR2 + R + R H 3.3.3. To empty orbitals: O + OEt 109 P OEt OEt isooctane Et + + Et P 109 O O Et Et isooctane Et B 109 Et isooctane 4. destruction of radicals OEt P OEt OEt Et P Et O Et O O + P OEt P OEt OEt Et + Et Et Et B Et Et O Et B + Et Et 2016January17 4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx 5 4.1. preceding reactions have “exchanged” radicals 4.2. coupling (recombination): R R R R 4.3. disproportionation: R'2 CH C H 2 R'2 CH C H 2 R'2 CHCH 3 R'2 C CH 2 4.4. oxidation: R Mn R M(n1) , M+n = Pb+4, Ce+4, Mn+3, Ag+2 5. chain reactions: path combination 5.1. example substitution: methane halogenation 5.2. propagation (chain reaction) can occur many times per initiation: 10 6 for Cl initiation propagation or h X 2 2X step F Cl Br I 1 -31 2 17 34 2 -70 -26 -24 -21 X CH4 XH CH3 CH3 X 2 CH3 X X termination Enthalpies of propagation steps X X X 2 5.3. termination rate constant is fast, but it is bimolecular and radical concentrations are low 5.4. propagation steps can compete because substrate concentration is high 5.5. feasibility determined by propagation steps, e. g. for methane bromination and iodination does not occur at an appreciable rate (rate related to enthalpy of propagation step, difference in bond energies 5.6. at 25 C, bromination of alkanes (per H): R3C-H (1600) > R2CH-H (80) > RCH2-H(1) 5.7. Chlorination of alkanes (per H): R3CH (6.7) > R2CH2 (4.4) > RCH3 (1) 5.8. polar effects- chlorination more sensitive than bromination 5.8.1. the -CH bonds are weakest but are also relatively electron deficient 5.8.2. the chlorine radical is electrophilic so it avoids the elecron deficient -CH CH3CH2CH2CN + Cl2 ClCH2CH2CH2CN + CH3CHClCH2CN + HCl 31% 69% 4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx Chlorination of chlorobutane: 6. radical addition 6.1. HBr addition: H (kcal/mol) of X addition and H abstraction steps X + CH2=CH2 XCH2CH2 + HX HF -45 37 HCl -26 5 HBr -5 -11 HI 7 -27 For halogen acids only HBr is exothermic for both reactions 6.2. RSH, Br3CH, Cl3CH are the same mechanism 6.3. BrCCl3, and CCl4 have a carbon or silicon radical initiation 2016January17 6 4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx 6.4. X2 6.5. Polymerization 6.5.1. Chain length determines concentrations and rates of steps 6.5.2. Branching can occur from H abstraction 7. addition to nitroso or nitrone compounds 7.1. stable radicals are formed 7.2. two-atom 3-electron bonds, what are MO’s for NO? R R N O + R' R' N O R' O O N N + R' 2016January17 7 2016January17 4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx 8. autooxidation Ri ROOH RO RO initiation (source of ROOH?) OH!!! RO RH ROH R R O2 RO 2 propagation RO 2 RH RO 2H R propagation 2RO 2 RO 4R termination 9. inhibitors - abstraction or addition forms unreactive radicals OH OH tBu O HO tBu 2R O R + O2 RO2 R RO2 OH Me O RO4R -tocopherol BHT hydroquinones O RSH - OH + N - O R + N N+ - O R tBu R - thiol O O + N O- tBu R ascorbate spin traps spin adducts 10. selected reactions 10.1. hydroxylation arenes H2O2 Fe2+ H2O2 HO - + HO + HO - + HO OH OH H H 10.2. silicon hydride (or tin hydride) reduction of alkyl halides.??? ROOR 2RO RO Et3 SiH ROH Et3 Si Et3 Si RX Et3 SiX R R Et3 SiH RH Et3 Si 10.3. diacyl peroxide reduction, catalytic in copper (only for R = alkyl) OH 8 2016January17 4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx 10.3.1. cation may rearrange or react with added nucleophile ast RCO 2 f R CO2 R Cu2 R Cu R RCO 2 RCO 2R 10.4. rearrangements 10.4.1. phenyl migration, delocalization provide low energy Ph Ph Ph Ph Ph Ph 10.4.2. Alkyl radical rearrangements rare! Compare carbon cation transition state or intermediate structure 10.5. Dissolving metal reduction 10.5.1. olefins, alkynes, aromatics 10.5.2. note that C radicals oxidize electropositive metals 10.5.3. RX + 2Na R2 9 2016January17 4311/6311 ch11-physical-organic-sykes-free-radical-reactions.docx H O R' Na + R R C C C C R H + Na R R R C C R + Na C C H R H O R' 11. esters, aldehydes and ketones (acyloin) + Na R C C H + Na + O R' R R C C H + Na + O R' H R 10