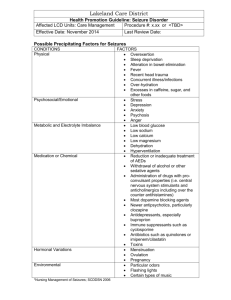
Supplementary Data
advertisement

Ictal HFOs identify seizure territories 1 Supplemental Data Figure S1: Action potentials are phase locked to high gamma oscillations. Representative traces of high gamma (red) and corresponding multi-unit activity (black) recorded from the penumbra (top) and the ictal core during (middle) and after (bottom) passage of the ictal wavefront. The phase angle distribution demonstrates particularly robust phase locking in the ictal core (right). Despite the stable amplitude of the multi-unit activity the amplitude of the high gamma oscillations was increased in the core relative to the penumbra. Note that in the ictal core, the high gamma oscillation is larger amplitude and leads the multiunit firing burst, suggesting that firing outside the listening sphere of the electrode is contributing. This indicates that high gamma currents have a larger driving force than action potentials, which would be necessary for detection of high gamma oscillations at the cortical surface. 1 Ictal HFOs identify seizure territories 2 Figure S2: Ictal multi-unit activity correlates with stronger high gamma oscillations in the ictal core than in the penumbra (a) Scatter plot of spike rate and high gamma amplitude during the preictal period (blue, r=0.52, slope=8.80, p<0.001), and during the subsequent full ictal activation (red, r=0.55, slope=43.96 p<0.001) in one seizure recorded from patient A. (b) Bar plot comparing the slope of preictal (blue) and ictal (purple, red) correlation between spike rate and high gamma power in both patients in which the seizure fully invaded the microelectrode array territory (red, n=4) and for seizures that did not invade the microelectrode array (purple, “penumbral activity”; n=6). 2 Ictal HFOs identify seizure territories 3 Figure S3: Examples of ictal high frequency oscillations (HFOs) recorded from the ictal core in subdural and micro-electrode recordings. Wideband tracings are shown for a segment of the ongoing ictal rhythm for each patient (top traces), paired with Morlet wavelet power-frequency spectrograms (bottom plots). The frequency and power scales were chosen in each case to 3 Ictal HFOs identify seizure territories 4 demonstrate the presence or absence of the signature characteristics of HFOs. (a) Ictal discharges recorded from a subdural electrode in the presumptive ictal core from patient C with superimposed HFOs visible in the raw signal. Note that superimposed HFOs are much smaller in amplitude than the high-amplitude low-frequency waveform, making them difficult to appreciate in the unfiltered signal. However, they are more easily appreciated in the spectrogram plots, which demonstrate “islands” with peak activity in the 80-150 Hz band. (b) Ictal discharges recorded from a subdural electrode in the presumptive ictal core from patient D (above). The HFOs are again difficult to discriminate visually, yet the corresponding Morlet wavelet transform demonstrates clear “islands”. (c) Ictal core discharges recorded from subdural electrodes in patient A (above). The discharges exhibit sharp transients, with visible HFOs and corresponding spectrogram “islands” evident during the discharges marked with a single asterisk (*). These contrast with high frequency activity lacking “islands” during discharges indicated by the double asterisk (**). Instead, these discharges produce a tapered cone appearance on the spectrogram, without a clear power peak in the high gamma band. (d) Ictal discharges recorded from a subdural electrode in the ictal core from patient B (above), also shown in Fig 2d. Again, both high frequency transients and spectrogram “islands” are evident. (e) Microelectrode recording from patient A of the same ictal discharges shown in panel C. The spectrogram islands of high gamma power during each discharge are now more clearly discerned. Note that the frequencies of these islands are higher than they are in subdural recordings. Simultaneously, increased power in the multi-unit activity band (300-3000 Hz) is evident. (f) A simulation consisting of a train of Gaussian waveforms with a half width similar to the ictal discharges recorded by subdural electrodes from patient A. Corresponding Morlet wavelet transform demonstrates energy spread over the entire frequency range, resulting in a tapered cone appearance, with no clear activity islands. 4 Ictal HFOs identify seizure territories 5 Figure S4: Examples of subdural and micro-electrode recordings lacking ictal high frequency oscillations. Unfiltered traces and corresponding Morlet wavelet spectrograms are shown as in Fig. S3. (a) Subdural recording from an electrode overlying the microelectrode array in patient C, a penumbral site (top). The spectrogram demonstrates scarce power in the high gamma band and activity resembling the tapered cone appearance described in Fig S3. (b) A similar subdural ictal recording from an electrode adjacent to the microelectrode array, again demonstrating a lack of distinct activity in the high gamma band. (c,d) Subdural recordings from electrodes 2 cm away from the microelectrode array in patients A and B, respectively, prior to incorporation into the ictal core based on the microelectrode-recorded multi-unit firing pattern and PLHG measure. Similar to the examples in panels A and B, the spectrogram demonstrates a lack of high gamma band activity, with a tapered-cone appearance showing energy spread over lower 5 Ictal HFOs identify seizure territories 6 frequencies. This is again consistent with effects of sharp transients, rather than distinct high frequency oscillations at the discharge peaks, which are less evident in the unfiltered traces. (e) Microelectrode recording from patient C, when multi-unit activity demonstrated heterogeneous firing characteristic of the penumbra. There are no islands of high gamma activity evident in the spectrogram, but there is sporadic power in the (300-3000 Hz) band corresponding to neural firing. 6 Ictal HFOs identify seizure territories Pre-recruitment 7 Recruitment Post-recruitment (n=203) ISI Penumbra (n=220) 42.29 +/- 3.13 12.72 +/- 0.53 31.69 +/- 1.11 333.27 +/- 36.40 ms ms ms ISI CV 2.60 +/- 0.06 1.56 +/- 0.02 1.51 +/- 0.02 1.55+/-0.33 % Electrodes 69.53 +/- 16.12% 72.20 +/- 11.1% 88.07 +/- 3.7 % 29.79 +/- 5.1% Z statistic 12.0 +/- 0.567 9.13 +/- 0.37 21.05 +/- 1.32 4.560+/-0.26 Mean phase 145.48+/-1.64 161.53+/-1.79 147.24 +/- 1.69 172.74+/-6.15, with MUA phaselocked to HG angle Supplementary Table S1: Action potentials are phase locked to the trough of the high gamma oscillation during the seizure. (a) Inter-spike interval (ISI), coefficient of variation (CV) of the interspike interval, and results of Rayleigh’s test (significance at p < 0.05, Z statistic) for circular non-uniformity for action potentials during each seizure stage and in the ictal penumbra. 7 Ictal HFOs identify seizure territories 8 R2 F p n A seizure 0.2631 31.4174 0.00001 90 B seizure 1 0.3813 56.1970 0.00001 93 B seizure 2 0.0309 3.789 0.0539 121 B seizure 3 0.0702 8.984 0.0033 121 (spike cross-correlation vs. subdural electrode PLV) Supplementary Table S2: High gamma cross frequency coupling in the subdural EEG overlying the multi-electrode array correlates with the multi-unit spiking cross correlation during the seizure. Results of linear regression analysis between multi-unit cross correlation (bottom) and subdural phase locking value for all 4 seizures captured by the microelectrode array in the ictal core. Supplementary Movies S1-S4: Mapping seizure activity using high frequency oscillations. Movies demonstrating dynamic changes in phase locked high gamma and line length during the initial time period of seizures in the four patients from whom seizures were recorded with the microelectrode array (small square) positioned below the subdural electrode grid, depicted schematically by circles. All grid electrodes were 3mm diameter, spaced 1 cm center to center. Ictal wavefront passage and subsequent core activity was detected by the microelectrode array in patients A (Movie S1) and B (Movie S2). The array recorded only penumbral activity in patients D (Movie S3) and C (Movie S4). The left panels in each movie depicts a line length measure used to approximate the arrival of the seizure at each electrode as judged by the visible range of EEG. Subthreshold values are indicated by the copper color scale, and channels meeting “recruitment” criteria (> 2.5 SD over preictal baseline x 2 seconds) are marked in solid blue. Similarly, PLHG values are shown in the right panels, with channels meeting threshold 8 Ictal HFOs identify seizure territories 9 marked in solid red. The movies show the dramatic contrast in the apparent seizure progression as indicated by the two criteria, with PLHG progression corresponding well with the timing (or failure) and direction of wavefront propagation as detected by the microelectrode array. At any given time, the extent of the seizure core is far smaller by PLHG criteria than it is by linelength criteria, an indication of the large extent of the penumbra and the degree to which it influences seizure localization. Supplementary Methods Subject enrollment and surgical procedures: Participants consisted of adults with pharmacoresistant focal epilepsy who underwent chronic invasive EEG studies to help identify the epileptogenic zone for subsequent removal. Enrollment was restricted to patients for whom the presurgical evaluation indicated good localization, and invasive recordings were required to refine the resection boundaries. The study was approved by the Institutional Review Board of the Columbia University Medical Center and informed consent was obtained by each patient prior to implantation. A 96 channel microelectrode, 4 mm×4 mm array recording from cortical layers III-V (Neuroport, Blackrock Microsystems Inc., Salt Lake City, UT) (House et al., 2006), was implanted along with subdural electrodes (Ad-Tech Medical Instrument Corp., Racine WI or PMT Corp., Chanhassen, MN). The individual microelectrodes were platinum coated silicon, protruding 1 mm from the array base, electrically insulated except for the terminal 70 m. Electrode impedance at manufacture was 322 138 k. The microelectrode array was implanted into neocortical gyri through the pia matter using a pneumatic insertion technique (Waziri et al., 2009). The implant site was selected from presurgical EEG studies, and eloquent cortical sites such as Broca’s area were avoided. In all four patients the microelectrode array implantation site was within the seizure onset zone and was subsequently surgically treated. Multi-unit activity analysis: Calculations were performed using in-house software (Matlab, Mathworks, Natick, MA) and FIELDTRIP (http://fieldtrip.fcdonders.nl/) (Oostenveld et al., 2011). To compare the multi-unit firing rate from each channel with local field potential power, the complete seizure and 10 second preictal epoch were divided into bins with a width of 200 ms. Power in each bin was computed by applying a multitaper FFT for spectral smoothing. The Spearman rank correlation coefficient and linear regression slope between spike rate and LFP power was calculated across all time bins. These comparisons were repeated for 9 Ictal HFOs identify seizure territories 10 power in frequencies 1-500 Hz, divided into 5 Hz frequency bins. Correlations between firing rate and high gamma power were computed by summating the power in the 80-150 Hz frequency bins. Normalized overall firing rate for each seizure was calculated from the summation of spikes for each channel in each bin across all bins. Interspike interval and the coefficient of variation of the interspike interval were calculated using the “spike train analysis techniques toolbox” (http://glab.bcm.tmc.edu/ signal_processing_techniques/signal_proc.html). To calculate the phase angle of neuronal firing with respect to the high gamma oscillation we applied the Hilbert transform to the microelectrode recording after band pass filtering the signal (80-150 Hz) and subsequently manually separating the seizure into penumbra, ictal 1 ∞ 𝑥[𝜏] wavefront, and ictal core epochs. The Hilbert transform is defined as y[t]=H(x[t])=𝜋 ∫−∞ 𝑡−𝜏 𝑑𝜏 and results in the analytic signal z[n]=a[n]exp(i is the instantaneous phase. We then used spike timing tspike to calculate the spike] of each action potential with respect to the high gamma oscillation. We calculated the first trigonometric moment of these phase angles using the equation m’=∑ 𝑒𝑥𝑝𝑖𝜙𝑠𝑝𝑖𝑘𝑒 = R𝑒𝑥𝑝𝑖𝜃 . Rayleigh’s Z-test for circular uniformity was calculated as Z=nR2. The probability that the null hypothesis holds was estimated as 𝑝 = 𝑒𝑥𝑝−𝑍 . Cross correlation of multi-unit activity was performed by calculating the spike rate in bin sizes of five ms for each active channel across a 10 second interval two minutes prior to seizure and during the entire seizure (D, C) or during seizure post-recruitment (B, A). Across all bins the cross correlation value was calculated between all channel pairs. Each cross correlation calculation included three bins before, and three bins after the bin of interest for the channel pair. To create a time series of multi-unit cross correlation, the seizure was further divided into 333 ms bins and multi-unit cross correlation across all electrodes was calculated as described above for each bin. Statistical significance of the cross correlation between channel pairs was performed with a bootstrapping method by circularly shifting the bins by a random amount (varying up to one bin) and calculating the 98th percentile of the Rayleigh Z statistic for 1,000 trials to determine the per-channel threshold. References 10 Ictal HFOs identify seizure territories 11 House PA, MacDonald JD, Tresco PA, Normann RA. Acute microelectrode array implantation into human neocortex: preliminary technique and histological considerations. Neurosurgical Focus 2006; 20: E4. Oostenveld R, Fries P, Maris E. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011; 2011:156869. Waziri A, Schevon CA, Cappell J, Emerson RG, McKhann GM II, Goodman RR. Initial surgical experience with a dense cortical microarray in epileptic patients undergoing craniotomy for subdural electrode implantation. Neurosurgery 2009; 64: 540–545. 11