HW2 - Uddingston Grammar School
advertisement
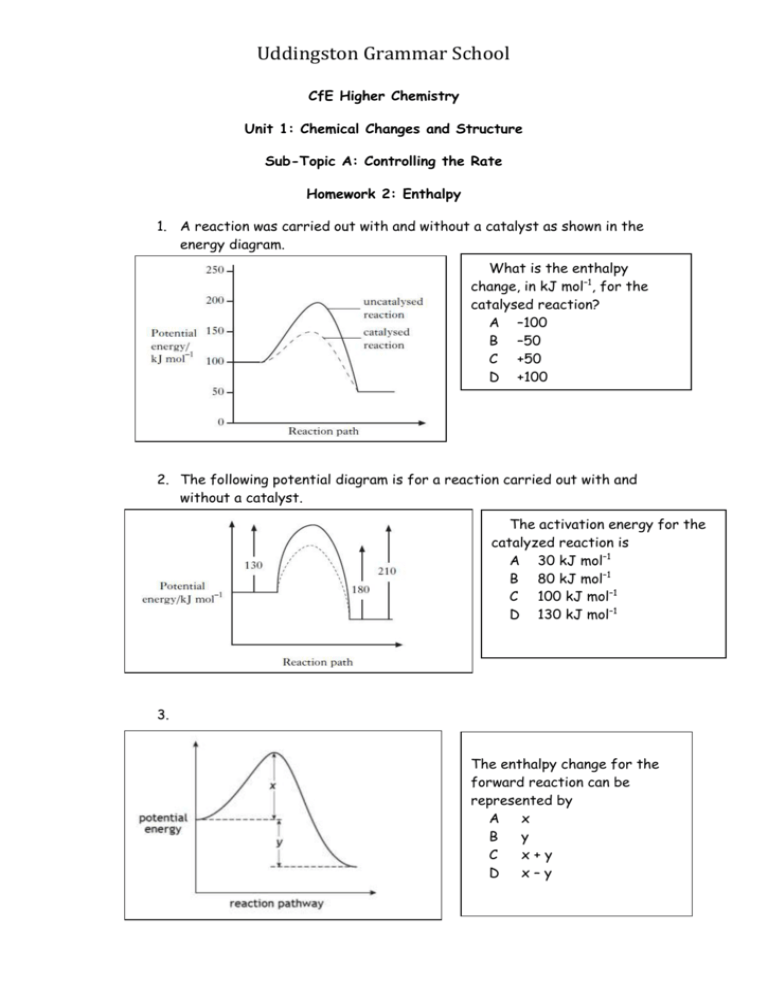
Uddingston Grammar School CfE Higher Chemistry Unit 1: Chemical Changes and Structure Sub-Topic A: Controlling the Rate Homework 2: Enthalpy 1. A reaction was carried out with and without a catalyst as shown in the energy diagram. What is the enthalpy change, in kJ mol–1, for the catalysed reaction? A –100 B –50 C +50 D +100 2. The following potential diagram is for a reaction carried out with and without a catalyst. The activation energy for the catalyzed reaction is A 30 kJ mol–1 B 80 kJ mol–1 C 100 kJ mol–1 D 130 kJ mol–1 3. The enthalpy change for the forward reaction can be represented by A x B y C x+y D x–y Uddingston Grammar School 4. A potential energy diagram can be used to show the activation energy (EA) and the enthalpy change (ΔH) for a reaction. Which of the following combinations of EA and ΔH could never be obtained for a reaction? A B C D EA EA EA EA = 50 kJ mol–1 and ΔH = –100 kJ mol–1 = 50 kJ mol–1 and ΔH = +100 kJ mol–1 = 100 kJ mol–1 and ΔH = +50 kJ mol–1 = 100 kJ mol–1 and ΔH = –50 kJ mol–1 5. Which set of data applies to the above reaction? Enthalpy change Activation energy/ kJmol –1 A Exothermic 60 B Exothermic 80 C Endothermic 60 D Endothermic 80 6. The following potential energy diagram represents the energy changes in a chemical reaction. The activation energy for the reaction, in kJ mol -1, is A 20 B 30 C 50 D 70 Uddingston Grammar School 7. The potential energy diagram below refers to the reversible reaction involving reactants R and products P. What is the enthalpy change, in kJmol-1, for the reverse reaction P A. B. C. D. R? +30 +10 –10 –40 8. When a catalyst is used, the activation energy of the forward reaction is reduced to 35kJmol-1. What is the activation energy of the catalysed reverse reaction? A. B. C. D. 30kJmol-1 35kJmol-1 65kJmol-1 190 kJmol-1 9. Which of the following diagrams represents an exothermic reaction which is most likely to take place at room temperature? Uddingston Grammar School Uddingston Grammar School 10. The potential energy diagram for the reaction is shown. CO(g) + NO2(g) → CO2(g) + NO(g) ΔH, in kJ mol–1, for the forward reaction is A +361 B –93 C –227 D –361 11. Which of the following is not a correct statement about the effect of a catalyst? The catalyst A B C D 12. provides energy so that more molecules have successful collisions lowers the energy that molecules need for successful collisions provides an alternative route to the products forms bonds with reacting molecules. Uddingston Grammar School In area X A B C D molecules always form an activated complex no molecules have the energy to form an activated complex collisions between molecules are always successful in forming products all molecules have the energy to form an activated complex 13. Which of the following describes the effect of a catalyst? Activation energy Enthalpy of reaction A decreased decreased B decreased no change C no change decreased D decreased increased 14. Soil bacteria are mainly responsible for releasing nitrogen in urea into the soil so that it can be taken up by plants. The first stage in the process is the hydrolysis of urea using the enzyme urease. (a) Determine the enthalpy change, in kJ mol−1, for the reaction. 1 (b) Acid is a less effective catalyst than urease for this reaction. Copy the graph and add a curve to the potential energy diagram to show the change in potential energy when acid is used as the catalyst. 1 Uddingston Grammar School 15. Enzymes are biological catalysts. (a) Name the four elements present in all enzymes. 1 (b) The enzyme catalase, found in potatoes, can catalyse the Decomposition of hydrogen peroxide. 2H2O2(aq) → 2H2O(l) + O2(g) A student carried out an experiment to determine the effect of pH on enzyme activity. Describe how the activity of the enzyme could be measured in this experiment. 1 (c) A student wrote the following incorrect statement. “When the temperature is increased, enzyme-catalysed reactions will always speed up because more molecules have kinetic energy greater than the activation energy.” Explain the mistake in the student’s reasoning. 1 16. Methanoic acid, HCOOH, can break down to carbon monoxide and water by two different reactions, A and B. Reaction A (catalysed) HCOOH(aq) + H+(aq) CO(g) + H2O(l) + H+(aq) Reaction B (uncatalysed) HCOOH(aq) heat CO(g) + H2O(l) (a) What is the evidence in the equation for Reaction A that the H+(aq) In acts as a catalyst ? 1 (b) Explain whether Reaction A is an example of heterogeneous or homogeneous catalysis. 1 Uddingston Grammar School 17. Nitrogen dioxide gas can be prepared in different ways. It is manufactured industrially as part of the Ostwald process. In the first stage of the process, nitrogen monoxide is produced by passing ammonia and oxygen over a platinum catalyst. NH3(g) + O2(g) → NO(g) + H2O(g) (a) Balance the above equation. (b) Platinum metal is a heterogeneous catalyst for this reaction. What is meant by a heterogeneous catalyst? (c) Explain how a heterogeneous catalyst works. TOTAL: 25 MARKS 1 1 3








