Heating Curve of Water
advertisement

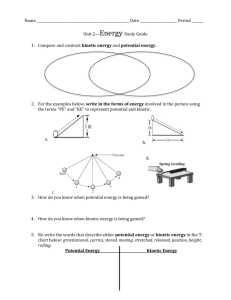
Heating Curve of Water Lab Activity Lab: Phase Change Chemistry: Students will investigate that the change from one phase of matter to another involves a gain or loss of energy. This will be done through measuring data and analyzing the constructed graph. Materials: 1. 2. 3. 4. 5. 6. 7. Hot plate 600-mL beaker Thermometer Thermometer clamp Ring stand 200 mL of distilled water Ice cubes Procedures: 1. Get approximately 200 ml of ice and 200 ml of water into the 600 ml beaker. 2. Set up a data table like the one provided to record both time and temperature. Record the temperature every 5 minutes. Start with 0 min. 3. Place the thermometer in the center, so that it does not touch the glass. 4. Collect data until you have at least 5 data points around 100°C and it does not change anymore. 5. Turn off the hotplate. Clean and organize your lab station. 6. Graph the data. Data: Table 1 Time (minutes) 0 min Temperature (Co) Create a temperature versus time graph and plot the corresponding data. Heating Curve of Water Lab Activity Analysis: 1. 2. 3. 4. 5. What is temperature? Why is the temperature going up? Why did the temperature remain constant around 100°C and creates a plateau? What is happening to the kinetic energy of the molecules as temperature rises? What would happen to the kinetic energy of the molecules if the temperature would be lowered? 6. How does this lab relate to kinetic molecular theory? Q&A 1. What is temperature? Temperature is a measure of the average energy of molecular motion in a substance. 2. Why is the temperature going up? . Higher temperatures mean that the molecules are moving, vibrating and rotating with more energy. They have higher kinetic energy. 3. Why did the temperature remain constant around 100°C and creates a plateau? The water is boiling and the particles are changing state from liquid to a gas. Essentially the temperature measured refers to the water (liquid state) molecules’ kinetic energy and not of the water vapor. Energy continuous to be added but the temperature stays the same because the water vapor’s kinetic energy is not taken into consideration. 4. What is happening to the kinetic energy of the molecules as temperature rises? As the temperature rises molecules move much for rapidly causing the kinetic energy to rise 5. What would happen to the kinetic energy of the molecules if the temperature would be lowered? If the temperature is lowered the particles will move slower causing the kinetic energy to lower. 6. How does this lab relate to kinetic molecular theory? The particles contain kinetic energy which is increased when thermal energy is applied. http://www.kentchemistry.com/links/Matter/HeatingCurve.htm Heating Curve of Water Lab Activity Essential question/Exit Slip