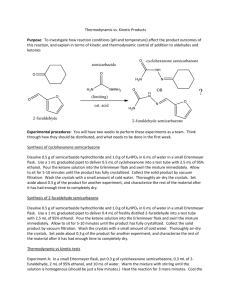
Kinetic vs Thermodynamic control
advertisement

Thermodynamic vs. Kinetic Products Purpose: To investigate how reaction conditions (pH and temperature) affect the product outcomes of this reaction, and explain in terms of kinetic and thermodynamic control of addition to aldehydes and ketones Experimental procedures: You will have two weeks to perform these experiments as a team. Think through how they should be distributed, and what needs to be done in the first week. Synthesis of cyclohexanone semicarbazone Dissolve 0.5 g of semicarbazide hydrochloride and 1.0 g of K2HPO4 in 6 mL of water in a small Erlenmeyer flask. Use a 1 mL graduated pipet to deliver 0.5 mL of cyclohexanone into a test tube with 2.5 mL of 95% ethanol. Pour the ketone solution into the Erlenmeyer flask and swirl the mixture immediately. Allow to sit for 5-10 minutes until the product has fully crystallized. Collect the solid product by vacuum filtration. Wash the crystals with a small amount of cold water. Thoroughly air-dry the crystals. Set aside about 0.3 g of the product for another experiment, and characterize the rest of the material after it has had enough time to completely dry. Synthesis of 2-furaldehyde semicarbazone Dissolve 0.5 g of semicarbazide hydrochloride and 1.0 g of K2HPO4 in 6 mL of water in a small Erlenmeyer flask. Use a 1 mL graduated pipet to deliver 0.4 mL of freshly distilled 2-furaldehyde into a test tube with 2.5 mL of 95% ethanol. Pour the ketone solution into the Erlenmeyer flask and swirl the mixture immediately. Allow to sit for 5-10 minutes until the product has fully crystallized. Collect the solid product by vacuum filtration. Wash the crystals with a small amount of cold water. Thoroughly air-dry the crystals. Set aside about 0.3 g of the product for another experiment, and characterize the rest of the material after it has had enough time to completely dry. Thermodynamic vs kinetic tests Experiment A: In a small Erlenmeyer flask, put 0.3 g of cyclohexanone semicarbazone, 0.3 mL of 2furaldehyde, 2 mL of 95% ethanol, and 10 mL of water. Warm the mixture with stirring until the solution is homogenous (should be just a few minutes.) Heat the reaction for 3 more minutes. Cool the reaction to room temperature, and in an ice bath until crystals form. Collect the crystals, wash them with 2-3 mL of ice cold water, and allow them to thoroughly dry. Obtain characterization data. Experiment B: In a small Erlenmeyer flask, put 0.3 g of 2-furaldehyde semicarbazone, 0.3 mL of cyclohexanone, 2 mL of 95% ethanol, and 10 mL of water. Warm the mixture with stirring until the solution is homogenous (should be just a few minutes.) Heat the reaction for 3 more minutes. Cool the reaction to room temperature and in an ice bath until crystals form. Collect the crystals, wash them with 2-3 mL of ice cold water, and allow them to thoroughly dry. Obtain characterization data. Experiment C. Dissolve 1.0 g of semicarbazide.HCl and 2.0 g of dibasic potassium phosphate in 25 mL of water. (This solution is about pH 6.) Cool to close to zero degrees in an ice bath. In a different container, dissolve 1.0 mL of cyclohexanone and 0.75 mL of 2-furaldehyde in 5 mL of 95% ethanol. Cool the solution to close to zero degrees in an ice bath. Add the ethanol solution into the aqueous solution while in the ice bath and swirl the mixture. Crystals should form quickly. Allow the reaction to sit in the ice bath for about 5 minutes, then collect the crystals by vacuum filtration. Wash them with 2-3 mL of ice cold water, and thoroughly air dry them. Collect mass and characterization data. Experiment D. Dissolve 1.0 g of semicarbazide.HCl and 2.0 g of dibasic potassium phosphate in 25 mL of water. (This solution is about pH 6.) Separately, dissolve 1.0 mL of cyclohexanone and 0.75 mL of 2furaldehyde in 5 mL of 95% ethanol. Add the ethanol solution into the aqueous solution at room temperature and swirl the mixture. Crystals should form within 1-2 minutes. Allow the reaction to sit at room temperature for about 5 minutes, then cool in an ice bath until crystallization is complete (5 minutes) . Collect the crystals by vacuum filtration. Wash them with 2-3 mL of ice cold water, and thoroughly air dry them. Collect mass and characterization data. Experiment E. Dissolve 1.0 g of semicarbazide.HCl and 2.0 g of dibasic potassium phosphate in 25 mL of water and warm the solution to about 80-85oC. (This solution is about pH 6.) Separately, dissolve 1.0 mL of cyclohexanone and 0.75 mL of 2-furaldehyde in 5 mL of 95% ethanol and warm the solution to about 80-85oC. Add the ethanol solution into the aqueous solution and swirl the mixture. Continue to warm the solution for 10-15 minutes, allow it to cool to room temperature, and then place the reaction to sit in the ice bath for about 5-10 minutes. Collect the crystals by vacuum filtration. Wash them with 2-3 mL of ice cold water, and thoroughly air dry them. Collect mass and characterization data. Experiment F. Dissolve 1.0 g of semicarbazide.HCl and 2.0 g of sodium bicarbonate in 25 mL of water. (This solution is about pH 7.) Separately, dissolve 1.0 mL of cyclohexanone and 0.75 mL of 2-furaldehyde in 5 mL of 95% ethanol. Add the ethanol solution into the aqueous solution at room temperature and swirl the mixture. Crystals should form within 1-2 minutes. Allow the reaction to sit at room temperature for about 5 minutes, then cool in an ice bath until crystallization is complete (5 minutes) . Collect the crystals by vacuum filtration. Wash them with 2-3 mL of ice cold water, and thoroughly air dry them. Collect mass and characterization data. Experiment G. Dissolve 1.0 g of semicarbazide.HCl and 2.0 g of sodium bicarbonate in 25 mL of water and warm the solution to about 80-85oC. (This solution is about pH 7.) Separately, dissolve 1.0 mL of cyclohexanone and 0.75 mL of 2-furaldehyde in 5 mL of 95% ethanol and warm the solution to about 8085oC. Add the ethanol solution into the aqueous solution and swirl the mixture. Continue to warm the solution for 10-15 minutes, allow it to cool to room temperature, and then place the reaction to sit in the ice bath for about 5-10 minutes. Collect the crystals by vacuum filtration. Wash them with 2-3 mL of ice cold water, and thoroughly air dry them. Collect mass and characterization data. Discussion Comments: What is a competition experiment? Explain with reference to the stoichiometry of experiments C-G. What is the advantage of running a competition experiment rather than comparing independently determined rate constants for two reactions? Give a one sentence purpose for why each experiment was run. What information is gained in each case that helps you to understand more about this reaction? Discuss the predicted reactivity/stability of the starting aldehyde and starting ketone based on their structures. Discuss the predicted reactivity/stability of the two final products based on their structures. Are these reactions under kinetic control or thermodynamic contraol? What evidence supports your assertion? How does change in temperature affect kinetic vs. thermodynamic control of these reactions? How does the change in pH affect kinetic vs. thermodynamic control of these reactions?