Dimensional Analysis - Moles Gases and Solutions Worksheet
advertisement

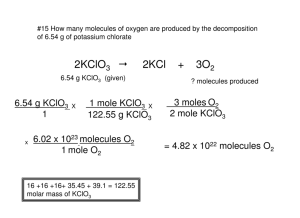
Dimensional Analysis – Mole, Gases and Solutions Worksheet Dimensional Analysis – Factor Labeling Method, Moles, Gases and Molarity 1. Make the following conversions: a. b. c. d. 31.2 L of CO2 gas under S.T.P. conditions to moles of CO2 0.456 mole of Cl2 gas to L of Cl2 gas under S.T.P. 2.3 L of 1.26 M NaCl solution to moles of NaCl 0.256 mole of H2SO4 to L of H2SO4 with the concentration of 1.24 M 2. Complete the following calculations: a. What is the concentration in molarity for a solution of CaCl2 that contains 1.45 x 102 moles of CaCl2 and a volume of 135 L? b. How many liters of SO2 gas under S.T.P. conditions is equivalent to 0.345 mole of SO2? c. How many moles of P2O5 is equivalent to 12.6 L of P2O5 gas under S.T.P. conditions? d. How many liters of FeCl3 solution at 1.25 M concentration is needed to produce 0.456 mole of FeCl3? 3. Answer the following fairly challenging questions. Remember using the factor-labeling protocol. Multiple bars may be needed. It is crucial to maintain a clear and precise description of units. a. How many grams of NaCl are there in 3.21L of a solution with concentration of 0.0525M? b. How many liters of 2.87 M CaCl2 solution is needed to produce 45.2 g of CaCl2? c. How many grams are there in 3.21 L of P2O5 under S.T.P. conditions? d. How many liters of SO2 gas under S.T.P. conditions are there in 32.6 g of SO2?