ONLINE DATA SUPPLEMENT
advertisement

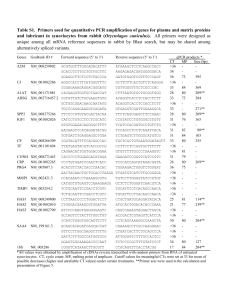
CCMED-D-11-01204 ONLINE SUPPLEMENT Crosstalk between calpain and caspase-3 during mechanical ventilationinduced diaphragmatic atrophy W. Bradley Nelson, Ashley J. Smuder, Matthew B. Hudson, Erin E. Talbert and Scott K. Powers CCMED-D-11-01204 METHODS and RESULTS In Vitro Protease Inhibition Assay. Often, studies using pharmacological inhibitors are criticized due to concerns about off-target effects of the pharmacological agents. To determine if our agents could successfully inhibit their target proteases while also not inhibiting other proteases believed to be important in mechanical ventilation (MV)-induced diaphragm weakness, we designed a series of in vitro experiments adapted from Pereira et al (1). Using purchased purified active enzymes (calpain-1, caspase-3 and caspase-9), we measured the cleavage of fluorogenic substrates when exposed to concentrations of inhibitor similar to our calculated in vivo concentrations, based on 70% body water. Sample fluorescence, an indicator of substrate cleavage, was measured after 20 minutes of incubating substrate, active enzyme, and the inhibitor. The calpain inhibitor (SJA-6017) prevented the majority of cleavage of the calpain substrate by active calpain-1, while our caspase-3 inhibitor (ACDEVD-CHO) did not prevent cleavage of the substrate (Figure 1). Similarly, after 20 minutes of incubation, the caspase-3 inhibitor (AC-DEVD-CHO) prevented cleavage of the caspase-3 substrate by active caspase-3, while the calpain inhibitor did not prevent substrate cleavage by caspase-3 (Figure 2). Incubation of the calpain inhibitor with active caspase-9 did not prevent substrate cleavage, while the caspase-3 inhibitor did in fact inhibit the activity of caspase-9 (Figure 3). Results from the in vitro assays are represented in Figures 1-3. CCMED-D-11-01204 Figure 1. Calpain-1 activity in the presence of the experimental inhibitors. Bars represent end-point fluorescence after 20 minute incubation. Sub = substrate, Calp-1 = purified calpain-1 enzyme, Calp Inhib = calpain inhibitor (SJA-6017), Casp-3 Inhib = caspase-3 inhibitor (AC-DEVD-CHO). Values are means ± SEM, groups with different letters are statistically different p<0.01, n=4. CCMED-D-11-01204 Figure 2. Caspase-3 activity in the presence of the experimental inhibitors. Bars represent end point fluorescence after 20 min incubation. Sub = substrate, Casp-3 = purified caspase-3 enzyme, Casp-3 Inhib = caspase-3 inhibitor (AC-DEVD-CHO), Calp Inhib = calpain inhibitor (SJA-6017). Values are means ± SEM, groups with different letters are statistically different p<0.01, n=4. CCMED-D-11-01204 Figure 3. Caspase-9 activity in the presence of the experimental inhibitors. Bars represent end point fluorescence after 20 min incubation. Sub = substrate, Casp-9 = purified caspase-9 enzyme, Casp-3 Inhib = caspase-3 inhibitor (AC-DEVD-CHO), Calp Inhib = calpain inhibitor (SJA-6017). Values are means ± SEM, groups with different letters are statistically different p<0.01, n=4. CCMED-D-11-01204 Standardization of protein loading for Western blotting. Ponceau-S stained membranes were used to control for equal protein loading and transfer differences. The membrane was scanned, and lanes were quantified to normalize Western blots to protein loading. Figure 4 provides a representative membrane that has been stained with Ponceau S. Figure 4. Representative Ponceau S stained membrane. 1, control; 2, 12 hr MV; 3, 12 hr MV + Calpain Inhibitor; 4, 12 hr MV + Caspase-3 Inhibitor. MW, molecular weight. CCMED-D-11-01204 Arterial blood gas and arterial blood pressure measurements. To ensure that our MV protocol was successful in maintaining homeostasis, we measured arterial pH, arterial blood pressures, heart rate, arterial PO2, arterial PCO2 and in all MV animals at the beginning of the experiments and at various time intervals during MV (see figure 5). The carotid artery was cannulated to allow for blood gas sampling and direct blood pressure monitoring via pressure transducer (Kent Scientific, Torrington, CT). Arterial blood gases and arterial blood pH were made with ~ 150 μl sample and measured via a clinical blood gas analyzer (GEM Premier 3000, Instrumentation Laboratory, Lexington, MA). Heart rates were monitored using a lead II electrocardiograph (model 2522B, BK Precision, Yorba Linda, CA). Figure 5. Hemodynamic results from mechanically ventilated animals. pH values represent range of values, all other values represent means ± SEM. CCMED-D-11-01204 References 1. Pereira NA, Song Z. Some commonly used caspase substrates and inhibitors lack the specificity required to monitor individual caspase activity. Biochem Biophys Res Commun 2008;377(3):873-877.