Amino Acid Metabolism Objectives
advertisement

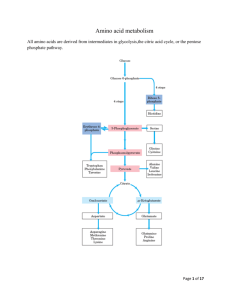
Amino Acid Metabolism: Objectives 1. Few questions… a. Which amino acids are the essential ones and which are nonessential? i. Essential – there are no biosynthetic pathways or not synthesized in adequate quantities 1. Arginine **Conditionally essential (can be made from glutamate + aspartate) 2. Histidine **Conditionally essential 3. Isoleucine 4. Leucine 5. Lysine 6. Methionine 7. Phenylalanine 8. Theronine 9. Tryptophan 10. Valine ii. Nonessential 1. Alanine (made from pyruvate and an amino acid) 2. Asparagine (made from aspartate + glutamine) 3. Aspartate (made from oxaloacetate) 4. Cysteine **Made from methionine (+ serine) 5. Glutamate (made from aspartate + alpha-ketogluterate) 6. Glutamine (made from glutamate and ammonia) 7. Glycine (made from threonine) 8. Proline (made from glutamate) 9. Serine (made from glutamate or glycine or choline) 10. Tyrosine**Made from phenylalanine b. Which nonessential amino acids are derived directly from an essential amino acid? i. They are readily made from intermediates of central pathways c. Which amino acids are conditionally essential and why? i. Arginine – not essential if have adequate glutamate and aspartate ii. Histidine – essential in infants (pathway is present as age) d. Methionine: need in higher amounts (solely responsible for making Cysteine) e. Phenylalanine: need in higher amounts (solely responsible for making Tyrosine) 2. How are amino acids absorbed in small intestine? a. Amino acids are taken up by enterocytes in the small intestine i. AAs transfer through enterocytes to the portal circulation by diffusion, facilitated diffusion, or active transport 3. What molecules serve as carriers of nitrogen? a. Glutamine (in a number of pathways) b. Alanine (inter-organ transfer of nitrogen…as in glucose-alanine cycle) c. Urea (transfers N in blood for excretion in the urine is its ONLY function) 1 Amino Acid Metabolism: Objectives 4. What properties of urea make it well suited as a carrier of nitrogen? a. Urea is nontoxic. b. Urea is highly water soluble. c. Its only purpose in life is to excrete excess Nitrogen out of the body and into the toilet. 5. Explain how transaminases and glutamate dehydrogenase play a central role in nitrogen metabolism. a. N is carried in blood serum as an amino group on amino acids i. Primarily, alanine and glutamine b. Transaminases: transfer amino groups to alpha-ketogluterate to make glutamate i. Amino Acid + alpha-ketogluterate keto acid + Glutamate c. Glutamate dehydrogenase: releases ammonia for urea synthesis i. Glutamate + NADP +H2O alpha-ketogluterate + NADPH + NH3 1. Enzyme: glutamate dhd d. Thus, a free NH3 is formed… which can be brought together with HCO3- and attach to urea to be excreted i. NH3 + HCO3- Urea to be excreted 6. Under what conditions are ammonium ions excreted in relatively high concentrations in the urine? a. During metabolic acidosis: i. Ex: Ketoacidosis due to uncontrolled diabetes mellitus is most common ii. Urea production is suppressed iii. Ammonium is excreted directly by the kidney! b. Glutamine + H2O Glutamate +NH4+ i. What enzyme plays an important role under these conditions? 1. Glutaminase 7. Explain how the glucose alanine cycle operates in inter-organ metabolism. Why is this cycle important? a. Provides for the nontoxic transport of nitrogen (produced by metabolizing amino acids) from skeletal muscle b. Produces pyruvate for the production of glucose by gluconeogenesis (in the liver) i. Especially useful in times of fasting or starvation Glucose Alanine Cycle Blood Amino acid keto acid Liver Muscle 2 Amino Acid Metabolism: Objectives 8. Explain how pyridoxal phosphate functions in amino group transfer by transaminases. a. Pyridoxal phosphate acts as an electrophile for covalent catalysis i. Essential for the transfer of N (as an amine group) that goes eventually to urea b. Pyridoxal phosphate and transaminase attach i. Then, transaminase and the amino acid switch places 1. So the pyridoxal phosphate is attached now to the amino acid ii. Release of the amino acid, and the free pyridoxal phosphate are produced. c. THUS: Pyridoxal phosphate acts as an electrophile 9. How are the reactions of the urea cycle compartmentalized inside of cells? a. Occurs in the Kidney and the Liver: i. Occurs within the mitochondria: 1. HCO3- + NH3 + 2 ATP Carbamoyl Phosphate a. Enzyme: Carbamoyl phosphate synthetase I 2. Ornithine + Carbamoyl phosphate Citrulline and PO4H2a. Ornithine needs a membrane carrier to enter or exit the mitochondria i. Ornithine is made in the cytosol ii. Occurs within the cytosol: 1. Citrulline + Aspartate + ATP Argininosuccinate a. Citrulline is transported into the cytosol (via membrane carrier) 2. Argininosuccinate Fumerate + Arginine b. In the liver, the reactions will continue: i. Arginine + H2O Ornithine + Urea NH3 + HCO3- Carbamoyl phosphate synthetase I 2 ATP 1 2 ADP + Pi Carbamoyl PO4 PI Ornithine transcarbamylase 2 Mitochondrial Matrix Urea Cycle Summary Aspartate Ornithine Citrulline Ornithine Citrulline Urea Argininase Aspartate 5 ATP 3 Agininosuccinate synthetase AMP + PPi H2O Arginine Cytosol Glutamate 4 argininosuccinate Fumarate Argininosuccinate lyase 3 Glutamate Amino Acid Metabolism: Objectives 10. Explain the role of the shuttles for ornithine/citrulline and malate/aspartate in the urea cycle. a. Glutamate is an anti-transporter with Aspartate i. Aspartate into the cytosol, glutamate into the mitochondria b. Alpha-ketogluterate is an anti-transporter with Malate i. Alpha-ketogluterate into the cytosol, malate into the mitochondria c. Citrulline and ornithine are antiports as well i. Citrulline into the cytosol, ornithine into the mitochondria d. See #9 diagram for more info… 11. How are the urea cycle and the TCA cycle linked? a. Malate (from Fumerate) b. Aspartate c. Alpha-ketogluterate formation (can enter at that point in the TCA) Links Between the Urea Cycle and the TCA Cycle α keto acid Aspartate Ornithine urea Citrulline Arginine α amino acid argininosuccinate Fumarate H2O fumerase Transaminase Aspartate Urea Cycle Oxaloacetate Citrate Malate Malate TCA Cycle Fumarate -Ketoglutarate Succinyl CoA 12. How is the urea cycle regulated? a. Long term regulation: i. Increased metabolism of AAs Increased synthesis of Urea cycle enzymes 1. Due to high protein diet (Carbon skeletons are oxidized and converted to fat or glycogen) 2. Due to starvation (Carbon skeletons from protein catabolism for energy are oxidized) b. Short term regulation: i. Arginine activates N-acetylglutamate (signals that high levels of AAs present) ii. Acetyl-CoA + glutamate N-acetylglutamate + CoA 4 Amino Acid Metabolism: Objectives 13. Explain why ammonia is toxic. Explain the elements of inter-organ metabolism that contribute to the self-perpetuating nature of ammonia intoxication. a. Ammonia is toxic, because when its concentration is high… i. Stimulates glucagon release from the pancreas 1. Which stimulates gluconeogenesis from AAs in the kidneys a. Results in even higher ammonium level i. Further stimulating glucagon release 1. Stimulate release of glucose (gluconeogenesis) a. Promotes insulin release i. Stim uptake of branched chain AAS by muscle b. Muscle uses AAs as oxidizable substrates 2. That metabolism causes release of more ammonium ions ii. In the brain, stimulates glutamine synthesis, promotes tryptophan transport b. Increase of tryptophan, increase the synthesis of 5-OHtryptamine (an important neurotransmitter) 2. Causing depletion of alpha-ketogluterate and glutamate in brain! b. It obviously has a cascading effect! Higher ammonia []causes even higher ammonia []! c. Impaired Mitochondrial Function with Ammonia Intoxication i. Increased Ca++ uptake, causing the pump to work harder (which requires ATP) ii. Decrease ETC activity, and thus the ATP production is slowed iii. Increased formation of free radicals and also NO iv. Increase in lipid peroxidation 14. What TCA cycle intermediate is depleted in the brain during ammonia intoxication and how does this happen? i. Alpha-ketogluterate is the TCA intermediate which is depleted in the brain ii. How it happens: See #13 for the cascade of happenings b. What amino acids are depleted in the brain? i. Alpha-ketogluterate ii. Glutamate c. What are the consequences of depletion of these metabolites for the brain? i. Inhibits TCA cycle and thus lowers ATP production, BUT increase in ATP need 15. How is ammonia intoxication treated and how do these treatments work? a. Diet: Low protein, high carb (which will reduce ammonia production) b. Levulose: Bacteria utilize this in colon a more acidic env. excrete NH4+ in the feces c. Antibiotics: Kills intestinal ammonia-producing bacteria in the gut d. Sodium Benzoate and Sodium Phenylbutyrate: form covalent products with glycine and glutamine; promoting N excretion in the feces 5 Amino Acid Metabolism: Objectives 16. Explain the distinction between a ketogenic vs a gluconeogenic fate of an amino acid. Which amino acids are exclusively ketogenic and to what are they ultimately metabolized? a. Ketogenic fate: Yield precursors which go to either fatty acids or ketone bodies i. Amino acids which are metabolized to Acetyl-CoA or Acetoacetyl-CoA ii. Amino acids which are exclusively ketogenic include: 1. Leucine: goes to Acetyl-CoA 2. Lysine: goes to alpha-ketoadipate, which goes to Acetyl-CoA b. Gluconeogenic fate AKA Glucogenic fate: amino acids which yield precursors that can be used for gluconeogenesis (can produce glucose) i. Metabolize to: 1. Pyruvate a. Include: Alanine, Cysteine, Serine, Threonine, Glycine 2. Alpha-ketogluterate a. Include: Arginine, Histidine, Glutamine, Proline 3. Succinyl-CoA a. Include: Methionine, Valine 4. Fumarate a. Include: Tyrosine 5. Oxaloacetate a. Include: Asparagine, Asparatate c. Both Ketogenic and glucogenic fates are possible i. Isoleucine: Acetyl CoA Propionyl CoA (Succinyl CoA) ii. Tyrosine: Acetoacetate Fumarate iii. Phenylalanine: Acetoacetate Fumarate 1. –forms into tyrosine iv. Tryptophan: Acetyl CoA Others… 17. To what TCA cycle intermediates are individual amino acids metabolized and where do these metabolites enter the TCA cycle. Alanine Serine Glycine Threonine Cysteine Tryptophan (through alanine) CO2 Pyruvate Acetoacetate Acetyl CoA Oxaloacetate Phenylalanine Tyrosine Tryptophan Lysine Citrate Asparagine Aspartate CO2 Fumarate Tyrosine Phenylalanine Aspartate Isoleucine Methionine Valine -Ketoglutarate Glutamate Succinyl CoA Methylmalonyl CoA CO2 6 Glutamine Proline Arginine Histidine Amino Acid Metabolism: Objectives 18. What metabolites accumulate in individuals with a defect in the branched chain keto acid dehydrogenase? What amino acids are metabolized by the pathway involving this enzyme? a. Branched Chain Keto Aciduria (AKA Maple Syrup Disease) i. Metabolites which accumulate: 1. Alpha-ketoiscaproate, alpha-keto-beta-methylvalerate, and alpha-ketoisovalerate ii. Enzyme with the defect is alpha-ketoacid(branched chain) dehydrogenase 1. AKA: BCKAD iii. Amino acids which are metabolized by the pathway involving BCKAD include: 1. Leucine, Valine, Isoleucine iv. Manifestation: severe mental retardation 1. Rare, recessive mutation isolated in Asian and Mennonite communities 19. What metabolites accumulate in alkaptonuria and what enzyme is defective in this disease? a. Accumulating metabolites include: i. Homogentisate 1. Causes urine to turn black on reaction with O2, at an alkaline pH ii. Phenylalanine iii. Tyrosine b. Enzyme: Homogentisate Oxidase i. Homogentisate Maleylacetoacetic acid c. Manifestation: damage to joints (arthritis), calcifications in the CVS, reddish tint (ochre) to the skin 20. How is S-adenosyl methionine synthesized? Synthesis of S-adenosyl Methionine S-adenosylmethionine (SAM) is an important methyl group donor. Methionine adenosyltransferase + ATP PPi + Pi S-adenosylmethionine (SAM or Adomet) methionine 7 Amino Acid Metabolism: Objectives 21. How is methionine regenerated from homocysteine? What coenzyme is involved in this reaction? What other enzyme catalyzed reaction in mammals utilized this coenzyme? a. N5-CH3 THF THF Methionine Synthase (B12 enzyme) CH3 group Methionine Homocysteine Adenosine SAM methyl group donor in many reactions; e.g.: Norepinephrine epinephrine Cytosine methylation of DNA b. Coenzyme: B12 c. B12 is also needed for Methylmalonyl CoA Succinyl CoS (along with the enzyme: Methylmalonyl-CoA Mutase) 22. What enzyme is defective in individuals with homocystinuria? What metabolite accumulates? How is this disease usually treated? a. Defective enzyme: Cysathionine Beta-Synthase i. Which is a vitamin B6 (pyridoxal phosphate) dependent enzyme b. Accumulating Metabolite: i. Homocysteine c. Treatment: i. If Vitamin responsive: treat with Vit B6 (pyridoxine), folic acid, vit B12 ii. If Vitamin Unresponsive: Restrict methionine 1. Supplement with cystine and betaine a. Promotes re-methylation of homocysteine to methionine 23. How are homocysteine thiolacetone and S-nitrosohomocysteine formed? a. They are formed from homocysteine’s reaction with protein amino groups i. Makes the modified protein immunogenic b. S-nitrosohomocysteine is formed from homocysteine and NO c. Homocysteine thiolacetone is formed from homocysteine i. By the enzyme: Methionyl-tRNA Synthetase 8 Amino Acid Metabolism: Objectives 24. Which amino acids can be made from intermediates of a central pathway and what intermediate gives rise to which amino acids? a. AAs made from intermediates of a central pathway: i. Transamination from a keto acid: 1. Pyruvate alanine 2. Alpha-ketogluterate glutamate 3. Oxaloacetate aspartate ii. Transamination of a keto acid followed by amidation: 1. Alpha-ketogluterate glutamate glutamine 2. Oxaloacetate aspartate Asparagine iii. Reductive Amidation: 1. Alpha-ketogluterate Glutamate (Enzyme: Glutamate Dhd) iv. From other intermediates of a central metabolic pathway: 1. Phosphoglycerate Serine 2. Phosphoglycerate Serine Glycine 3. Citrulline Argininorsuccinate Arginine v. From other amino acids: 1. Phenylalanine Tyrosine 2. Glutamate Proline 3. Methionine Cysteine 25. Explain the role of the small intestine and kidney in the synthesis of arginine. a. Synthesis of arginine begins in the enterocytes of the small intestine i. Glutamine y-semialdehyde + Glutamate Ornithine Citrulline ii. Citrulline is carried by the circulation to the kidney b. Synthesis of arginine continues in the kidney i. Citrulline+ Aspartate + ATP Argininosuccinate Fumerate + Arginine ii. Enzymes: 1st – argininosuccinate synthetase 2nd – argininosuccinate lyase c. The kidney lacks arginase of the urea cycle i. Thus, the kidney is a major site of arginine production d. This pathway is AKA “Intestinal-Renal Axis” 26. What reaction is catalyzed by phenylalanine hydroxylase and what is the role of tetrahydrobiopterin in this reaction. What is phenylketonuria? a. Phenylalanine Tyrosine Enzyme: Phenylalanine hydroxylase b. Tetrahydrobiopterin is the substrate (along with phenylalanine) for the formation of tyrosine i. Needs to be produced from dihydro-biopterin + NADH 1. Enzyme: Dihydropteridine Reductase c. Phenylketonuria: Defect in a phenylalanine hydroxylase gene i. Occasionally, this defect involves the dihydrobiopterin cofactor ii. Tx: Supplement with tyrosine, avoid phenylalanine 9 Amino Acid Metabolism: Objectives 27. How is nitric oxide made? What are some of the functions of this short-lived molecule? a. Arginine + O2 + NADPH Citrulline + NADP+ + NO b. Functions: i. iNOS (AKA NOSI): Immune and inflammatory responses ii. nNOS (AKA NOSII): Neurotransmitter in the GI tract, in penile erection, and in sphincter relaxation - acts on smooth muscle iii. eNOS (AKA NOSIII): Acts on endothelial cells; blood flow, blood pressure, and platelet activation Citrulline Nitric oxide Cycle ATP AMP + PPi Citrulline ASS Aspartate Citrulline NO Argininosuccinate NOS Arginine ASL Fumarate Just say NO. O2 ASS – Argininosuccinate synthetase c. ASL – Argininosuccinate lyase NOS – Nitric oxide synthase 10