File
advertisement

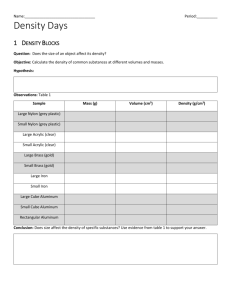
Magic Eggs Density and Buoyancy Teacher: Christina Bower Subject: Science Objective: Students will… apply the processes of scientific investigation and design, conduct, communicate about, and evaluate such investigations Content Objective: Which is more buoyant: saltwater or freshwater? How does salt affect buoyancy? How does the amount of salt added to water affect its buoyancy? Why? Materials (per pair): 2 extra large plastic cups filled with water (clear cups) 3 Tbsp. sea salt or kosher salt in a plastic bag 1 hard-boiled egg One drop of food coloring 2 “Egg-periment” worksheets Purpose: Students will discover the definitions of density and buoyancy by completing a hands on experiment with eggs and water. Background Information: Density affects buoyancy. Buoyancy is the ability of a liquid (such as water) to support or hold up other liquids or objects. Buoyancy can be measured as the mass of material that can be supported by one milliliter (1 ml) of liquid. For example, in fresh water, 1 gram of material can be supported by 1 ml of water; therefore the buoyancy of fresh water is 1 gram per ml (1 g/ml). The denser an object the less likely it will float. In a salt water solution, the egg is less dense than the salt water and it easily floats. In tap water, the egg is denser and therefore does not float. Density is calculated by dividing an objects mass by its volume. When measuring the density of a liquid, you have to take into account the mass of the container. All students need to use the same size containers and measuring cups to get similar answers about density. Students must be told not to eat the salt or the eggs because they will not be sanitary. They must also be cautious when using glass containers and quickly report any breakages to the teacher. Guided Questions: Review what was learned last class. Ask: What does buoyant mean? (Something is buoyant if it can float, i.e. buoyant means able to float). Can you name some buoyant objects? What do you think caused that object to float in water? Explain to students that today, we will be putting eggs in cups of water with and without salt. We will be observing and recording what happens to the egg. Ask: What do you think might happen to the egg in the cup of water? Listen to responses. We are going to fill our cups 3 ways. 1- with water, 2- some salt and water, and 3- a lot of salt and water.” Fill all 3 cups ¾ full with water in front of the students. Now, on your lab report, write your hypothesis I hypothesize that when I put an egg in plain fresh water it will ______________ because ______________________________. When I dissolve salt in the water I think the egg will _________________ because_______________________________. Exploration – Part One: After their hypotheses are written, dismiss them to their tables. Give the table manager the cups and salt. Then have the table managers come get the table’s water. Now place a raw egg in cup #1 and observe. Record what you observed and also draw a picture of the egg in the cup on your sheet. Put 1 tablespoon of salt in cup #2. Stir. Now place the egg in cup #2 and observe. Record what you observed and also draw a picture of the egg on your sheet. Now put 2 tablespoons of salt in cup #3. Place the egg in cup #3 and observe. Record what you observed and also draw a picture of the egg on your sheet. If we wanted to get the egg to suspend itself in the middle of the cup of water, what would we have to do? See if you are able to do it. Debrief: “What are your observations, recordings, and evidence? Please share. Think/Pair/Share “Why do you think this happens?” (The density of the water is changed causing objects to be more buoyant). Write your conclusion statements by answering the big questions: Which is more buoyant: saltwater or freshwater? How does salt affect buoyancy? How does the amount of salt added to water affect its buoyancy? Why do you think this happens? Exploration – Part Two: Distribute food colouring to each station. Tell the students they will be dying the freshwater and they may place about five drops of dye into the freshwater and stir it so the dye is even. Add more dye if the water is not dark enough. NO dye goes into the saltwater cup. Next, tell the students that they will be adding the dyed freshwater to the saltwater. Have students make a prediction on their Egg-periment worksheet as to what will happen to the dyed water when it is added to the saltwater cup. Have students slowly add the fresh water to the salt water. (The dyed freshwater should float on top). Students should record what happens on their worksheet by coloring it onto the template cups drawn on their worksheets. Last, have students clean up their areas and wash their hands. Discussion Have students join you on the floor for a discussion of the results. Ask questions that may lead to discussion about weight and density like: Were the results the same or different from your prediction? When does the egg sink? Why? Does the dyed freshwater float? Why? What is different about the saltwater as compared to the freshwater? Finally, ask if they think there is a place in nature where freshwater and saltwater are in the same place, and the freshwater stays on top. When salts are added to water, the water becomes more dense. Salt water then, is heavier than fresh. In an estuary, where salt and fresh water meet, the different densities cause the waters to settle into two layers; a salt water layer on the bottom with a freshwater layer above. Mixing occurs where the layers meet. Further mixing takes place as a result of wind, tides, waves, temperature changes and rainfall.