Linagliptin Drug Monograph - Internal
advertisement

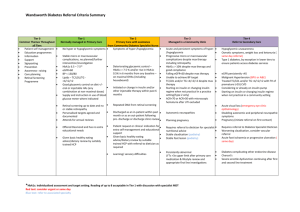
Drug Monograph Tradjenta® Linagliptin September 10th, 2012 Introduction Linagliptin, a novel xanthine-derived dipeptidyl peptidase (DPP)-4 inhibitor, was approved by the FDA on May 2nd, 2011. It is the newest addition to the DPP-4 inhibitor class and has demonstrated safety and efficacy in eight double-blind, placebo-controlled clinical studies involving approximately 3800 patients. Pharmacology DPP-4 inhibitors lower blood glucose through enhancement of glucose-dependent insulin synthesis and secretion by preventing DPP-4-mediated degradation of endogenous incretin hormones, like glucagon-like peptide (GLP)-1 and glucose-dependent insulinotropic peptide (GIP). Besides their actions on insulin, incretin hormones also regulate glucose homeostasis through inhibition of glucagon secretion, thereby decreasing glucose production. In addition to providing better glucose control, research has shown evidence of DPP-4 inhibitors improving β islet cell function as well as α cell sensitivity to glucose. Pharmacokinetics Absorption Absolute bioavailability of ~30%. May be administered with or without food Tmax ~1.5 hours postdose Distribution Vd is approximately 1,110 L (extensively distributed to tissues) Relatively high protein binding of 75-99% that varies depending on the drug Protein binding concentration Minor elimination, with only a small fraction metabolized to an inactive Metabolism metabolite Majority (~90%) is excreted unchanged with ~80% eliminated via the Elimination enterohepatic system and ~5% eliminated via the urine Effective t1/2 of ~12 hours with a biphasic decline in plasma concentration and a Half-life long terminal t1/2 of >100 hours. FDA Approved Indication(s) Type 2 diabetes mellitus: As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus as monotherapy or combination therapy. Dosage and Administration The usual dosage for T2DM is 5mg once daily. However, when used in combination with a sulfonylurea or insulin, a lower dose may be required of the sulfonylurea or insulin to decrease the risk of hypoglycemia. Special Populations Renal function impairment: No dose adjustment is recommended. Hepatic function impairment: No dose adjustment is recommended. Elderly: No dose adjustment is recommended. Children: Safety and effectiveness have not been established. Adverse Effects Hypoglycemia: 15% combined with metformin/sulfonylurea <1% combined with metformin, pioglitazone <1% monotherapy Other system adverse events: CNS Headache (6%) Hyperuricemia (3%), lipids increased (3%), triglycerides increased (2%), Endocrine & metabolic weight gain (2%) Neuromuscular & skeletal Arthralgia (6%), back pain (6%) Respiratory: Nasopharyngitis (6%), cough (2%) <1% (Limited to important or life-threatening): Angioedema, hypersensitivity, and pancreatitis Monitoring Parameters HbA1c and serum glucose Pregnancy/Lactation Considerations Pregnancy: Category B Lactation: Excretion in breast milk unknown, use caution Precautions/Contraindications Contraindications: Hypersensitivity to linagliptin or any component of the formulation Precautions: Concomitant use of insulin/sulfonylurea may increase the risk of hypoglycemia. Monitor blood glucose closely; dosage reduction of insulin/sulfonylurea may be required. Linagliptin should not be used in patients with DKA or type 1 diabetes mellitus due to lack of efficacy. Drug Interactions Linagliptin is a substrate of CYP3A4 and p-glycoprotein. Interacting Drug Class Example Description CYP3A4 strong inducers Rifampin May decrease linagliptin’s therapeutic effect. Ritonavir Linagliptin’s pharmacologic effects and adverse CYP3A4 strong inhibitors Ketoconazole reactions may be increased. Efficacy/Clinical Trial Citation Purpose Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomised controlled trial. Diabetes Obes Metab. 2011;13:258–267. To assess the safety and efficacy of linagliptin 5 mg when given for 24 weeks to patients with type 2 diabetes who were either treatment-naive or who had received one oral antidiabetes drug (OAD). Methods Criteria Results Study Design: Randomized, double blind, parallel-group study including patients enrolled from 66 trial sites in 11 countries. Following a 2 weeks placebo run-in for patients not pre-treated, and a 6 weeks washout period with the last 2 weeks a placebo run-in for patients pre-treated with 1 OAD, eligible patients were randomized to either treatment with linagliptin 5 mg or placebo (2:1 ratio) for 24 weeks The primary endpoint was change from baseline in HbA1c after 24 weeks of treatment (adjusted for baseline HbA1c and previous OAD). Secondary endpoints: absolute response, relative response, reduction from baseline in HbA1c by visit over time, change from baseline in fasting plasma glucose (FPG), and MTT(change in 2-hr postprandial glucose from baseline). The safety criteria were incidence and intensity of adverse events, withdrawals because of adverse events, physical examination, 12-lead electrocardiogram, vital signs and clinical laboratory parameters. Data Analysis The primary endpoint was assessed using analysis of covariance (ANCOVA) at the level of α = 0.05 (two-sided) based on the full analysis set (FAS). Inclusion 18-80 years with a BMI≤40 kg/m2 HbA1c between 6.5 and 9.0% in pre-treated patients and between 7.0 and 10% in treatment-naïve patients. (An HbA1c between 7.0-10.0% in both groups was required at the start of the run-in period. Exclusion Myocardial infarction, stroke, or transient ischemic attack within 6 months of study enrollment Impaired hepatic function at screening Receiving rosiglitazone, pioglitazone, GLP-1 analogues, insulin or antiobesity drugs (e.g. sibutramine, rimonabant or orlistat) within 3 months of enrolment. Patients receiving systemic steroids at enrollment or receiving dose changes in any thyroid hormone treatment within 6 weeks of screening. Efficacy The adjusted mean difference in the change inHbA1c comparing linagliptin and placebo was −0.69% (p < 0.0001). Linagliptin treatment resulted in a greater reduction of FPG (adjusted mean change −1.3 mmol/l; p < 0.0001) and 2hPPG (adjusted mean change −3.2 mmol/l; p < 0.0001) compared with placebo after 24 weeks. The improvement in glycaemic control achieved with linagliptin was associated with enhancement of markers of β-cell function, such as proinsulin/insulin ratio, HOMA-%B, and DI. there was no difference in the mean linagliptin trough levels over time between patients with normal renal function and those with mild or moderate renal impairment, 8.0 ± 7.3, 8.0 ± 7.6 and 6.6 ± 1.8 nmol/l, respectively. Conclusion Critique Safety The most frequently reported adverse events (frequency >2%) that were more common with linagliptin than placebo were headache (2.7 vs. 1.2%, respectively), hypertension (3.6 vs. 1.2%, respectively) and back pain (2.7 vs. 1.8%, respectively). Hyperglycemia was the most common adverse event, occurring in 8.6% of the linagliptin group and in 22.8% of the placebo group. Neither body weight nor waist circumference differed significantly from baseline in either group, confirming that linagliptin is weight neutral. Monotherapy with linagliptin produced a significant, clinically meaningful and sustained improvement in glycemic control, accompanied by enhanced parameters of β-cell function. The safety profile of linagliptin was comparable with that of placebo. Strengths Large trial that included 11 countries and 66 trial sites Blinded randomization Included renal function and drug concentration evaluation since it is the main clinical difference about linagliptin vs. other DPP-4 inhibitors Limitations No comparison to standard therapy/other DPP-4 inhibitors Washout period was only 6 weeks for those who had received a prior OAD, which effects of treatment on HbA1c may last up to 12 weeks Short duration of study period and thus inability to assess the long term effect of chronic treatment on glycemic control Ethical concerns of using a non-first line agent as monotherapy in T2DM even though its class effect on HbA1c has been shown to be minimal Cost The average wholesale price (AWP) of linagliptin is $8.12 per tablet; therefore, the estimated cost of a 30-day supply is $243.60. The AWP of a 30-day supply of sitagliptin or saxagliptin is similar to that of linagliptin. Conclusion Linagliptin has been studied in multiple studies, as monotherapy and as adjunct therapy with patients inadequately controlled on metformin, pioglitazone, or a sulfonylurea. Like the other DPP-4 inhibitors, it produces significant but minor reductions in HbA1c, thereby gaining a place as adjunct therapy rather than monotherapy in patients with T2DM. Also like its predecessors, its side effects profile is comparable to placebo and rarely causes hypoglycemia, but should be used with caution if it is combined with insulin or a sulfonylurea. However, unlike its predecessors, including sitagliptin and saxagliptin, it does not require dose adjustment for renal impairment because it is minimally eliminated via the renal route. This might give linagliptin substantial advantage over other DPP-4 inhibitors since renal impairment is a gradual process that accompanies the diabetic population. Linagliptin can also be used without dose adjustment in hepatic dysfunction. In regard to cost, linagliptin is similar to sitagliptin, the DPP-4 inhibitor most commonly used and the one on LSU-Shreveport’s formulary. The only limiting factor that might put linagliptin at a disadvantage to sitagliptin is its drug interaction profile due to it being a substrate of CYP3A4, the major metabolizing enzyme for many drugs. Recommendation Considering the efficacy, safety, and cost of linagliptin vs. sitagliptin, linagliptin should be added to the LSU-Shreveport formulary. The main advantage of doing so would be to offer diabetic patients a safe and effective alternative to use in the event of renal impairment without having to worry about adjusting the dose. References 1. FDA News Release: FDA Approves New Treatment for Type 2 Diabetes. FDA, US Food and Drug Administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm253501.htm. Updated: May 2, 2011. Accessed: September 8, 2012. 2. Baetta R, Corsini A. Pharmacology of Dipeptidyl Peptidase-4 Inhibitors, Similarities and Differences. Drugs. 2011;71:1441-1467. 3. UpToDate Inc. http://www.uptodate.com/. Accessed September 9, 2012. 4. Facts & Comparisons. http://online.factsandcomparisons.com. Accessed September 9, 2012. 5. Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type-2 diabetes: A randomized controlled trial. Diabetes Obesity Metab. 2011;13:258–267. 6. Freeman MK. Efficacy and Safety of Linagliptin (Tradjenta) in Adults With Type-2 Diabetes Mellitus. P T. 2011;36(12):807-812, 842.