View
advertisement
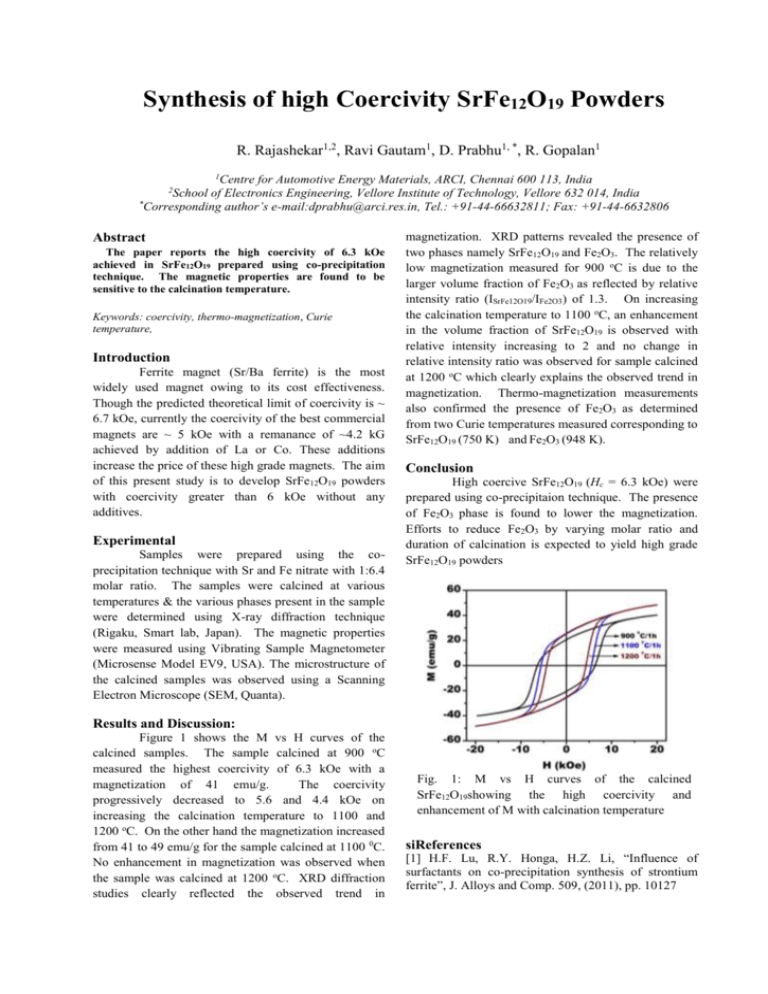
Synthesis of high Coercivity SrFe12O19 Powders R. Rajashekar1,2, Ravi Gautam1, D. Prabhu1, *, R. Gopalan1 1 Centre for Automotive Energy Materials, ARCI, Chennai 600 113, India School of Electronics Engineering, Vellore Institute of Technology, Vellore 632 014, India * Corresponding author’s e-mail:dprabhu@arci.res.in, Tel.: +91-44-66632811; Fax: +91-44-6632806 2 Abstract The paper reports the high coercivity of 6.3 kOe achieved in SrFe12O19 prepared using co-precipitation technique. The magnetic properties are found to be sensitive to the calcination temperature. Keywords: coercivity, thermo-magnetization, Curie temperature, Introduction Ferrite magnet (Sr/Ba ferrite) is the most widely used magnet owing to its cost effectiveness. Though the predicted theoretical limit of coercivity is ~ 6.7 kOe, currently the coercivity of the best commercial magnets are ~ 5 kOe with a remanance of ~4.2 kG achieved by addition of La or Co. These additions increase the price of these high grade magnets. The aim of this present study is to develop SrFe12O19 powders with coercivity greater than 6 kOe without any additives. Experimental Samples were prepared using the coprecipitation technique with Sr and Fe nitrate with 1:6.4 molar ratio. The samples were calcined at various temperatures & the various phases present in the sample were determined using X-ray diffraction technique (Rigaku, Smart lab, Japan). The magnetic properties were measured using Vibrating Sample Magnetometer (Microsense Model EV9, USA). The microstructure of the calcined samples was observed using a Scanning Electron Microscope (SEM, Quanta). magnetization. XRD patterns revealed the presence of two phases namely SrFe12O19 and Fe2O3. The relatively low magnetization measured for 900 oC is due to the larger volume fraction of Fe2O3 as reflected by relative intensity ratio (ISrFe12O19/IFe2O3) of 1.3. On increasing the calcination temperature to 1100 oC, an enhancement in the volume fraction of SrFe12O19 is observed with relative intensity increasing to 2 and no change in relative intensity ratio was observed for sample calcined at 1200 oC which clearly explains the observed trend in magnetization. Thermo-magnetization measurements also confirmed the presence of Fe2O3 as determined from two Curie temperatures measured corresponding to SrFe12O19 (750 K) and Fe2O3 (948 K). Conclusion High coercive SrFe12O19 (Hc = 6.3 kOe) were prepared using co-precipitaion technique. The presence of Fe2O3 phase is found to lower the magnetization. Efforts to reduce Fe2O3 by varying molar ratio and duration of calcination is expected to yield high grade SrFe12O19 powders Results and Discussion: Figure 1 shows the M vs H curves of the calcined samples. The sample calcined at 900 oC measured the highest coercivity of 6.3 kOe with a magnetization of 41 emu/g. The coercivity progressively decreased to 5.6 and 4.4 kOe on increasing the calcination temperature to 1100 and 1200 oC. On the other hand the magnetization increased from 41 to 49 emu/g for the sample calcined at 1100 0C. No enhancement in magnetization was observed when the sample was calcined at 1200 oC. XRD diffraction studies clearly reflected the observed trend in Fig. 1: M vs H curves of the calcined SrFe12O19showing the high coercivity and enhancement of M with calcination temperature siReferences [1] H.F. Lu, R.Y. Honga, H.Z. Li, “Influence of surfactants on co-precipitation synthesis of strontium ferrite”, J. Alloys and Comp. 509, (2011), pp. 10127