density of states

1.16 ENGINEERING PHYSICS - II
DENSITY OF STATES
A parameter of interest in the study of conductivity of metals and semiconductors is the density of states.
The Fermi function F(E) gives only the probability of filling up of electrons in a given energy state. It does not give the information about the number of electrons that can be filled in a given energy state, to know that we should know the number of available energy states called density of states.
Density of states is defined the as the number of energy states per unit volume in an energy interval of a metal. It is use to calculate the number
of charge carriers per unit volume of any solid.
N(E) dE =
Number of energy states between E and E + dE
Volume of the metal
N(E) dE =
D(E) dE
V
... (1) n z dn
E+dE
E n
O n y n x
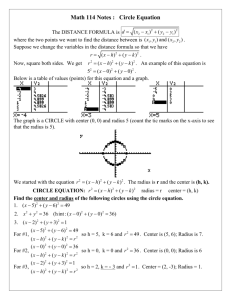
Fig.1.6 Positive octant of n space
Let us constant a sphere of radius “n” in space with quantum numbers n n , n y and n z n
2
= n x
n y
n z
The sphere is further divided into many shells represents a particular combination of quantum numbers and represents particular energy value.
CONDUCTING MATERIALS 1.17
Therefore, the number of energy states within a sphere of radius n =
4
n
3
3
Let us consider two energy values E and E + dE can be found by finding the number of energy states between the shells of radius n and n+ dn from the origin.
Since the quantum numbers are positive integers, n values can be defined only in the positive octant of the n – space.
The number of available energy states within the sphere of radius “n” due to one octant. n
1
4
8 3
n
3
Similarly the number of available energy states within the sphere of radius n+dn corresponding energy. n + dn =
1
4
8
n + dn
3
The number of available energy states between the shells of radius n and n + dn
(or) between the energy levels E and E + dE
D (E) dE =
1
8
4
n + dn
3
4
n
3
The number of available energy states between the energy interval dE
D (E) dE =
1
4
8
3
n
3
+ dn
3
+3n
2 dn+3n dn
2 – n 2
Since the higher powers of dn is very small, dn 2 and dn 3 terms can be neglected.
D (E) dE =
D (E) dE =
1
4
8
n
2
2
dn
3n
2 dn
... (3)
1.18 ENGINEERING PHYSICS - II
We know that the allowed energy values is n
2 h
2
E =
8mL
2
Differentiating equation (4) with respect to ‘n’ dE = ndn =
From equation (4) n
2
= h
2
8mL
2
2 ndn
8mL
2
2h
2
8mL
2
E dE h
2 n =
8mL
2
E
1/ 2
h
2
1/ 2
On substituting equation (6) and (5) in equation (3) we get,
n (ndn)
D (E) dE =
2
D (E) dE =
8mL
2
E
1/ 2
2
h
2
1/ 2
8mL
2
2h
2 dE
... (4)
... (5)
... (6)
D (E) dE =
8mL
4
h
2
2
3 / 2
3 / 2
E
1/ 2 dE
D (E) dE =
3/ 2 3 1/ 2
8m L E dE
4h
3
If volume of the metal, V = L
3
D (E) dE =
4h
3
3/ 2 1/ 2
8m VE dE
CONDUCTING MATERIALS 1.19
For unit volume of a metal,
V = 1m
3
From equation (1) N (E) dE =
Density of States, N (E) dE =
D(E) dE
V
3/ 2 1/ 2
8m E dE
4h
3
Each electron energy level can accommodate two electrons as per Pauli’s exclusion principle. (Spin up and Spin down = 2 (e) × density of states).
N (E) dE = 2 × N(E) dE
N (E) dE =
3 / 2 1/ 2
2 x
4h
3
8m E dE F(E)
N (E) dE =
3 / 2 1/ 2
8m E dE F(E)
2h
3
CONDUCTING MATERIALS 1.19