Alison Poulson DETERMINING THE MOST EFFICIENT ICE
advertisement

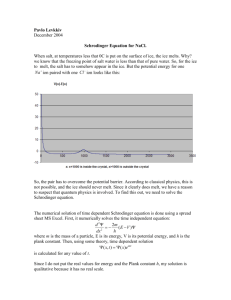
Alison Poulson DETERMINING THE MOST EFFICIENT ICE-MELT Introduction In much of North America, winter snowfall causes accidents and injuries. To minimize these problems, cities shovel roads and use ice-melt to keep them clear. Ice-melt is usually a salt and lowers the melting point of H2O by changing the concentration of ions suspended in it. Any water-soluble substance will lower the melting point of ice, but for ice-melt to be effective the depressed melting point must be lower than the air temperature. There are many factors involved in choosing a commercial ice-melt, but a key question is whether dissolving the substance is an endothermic or an exothermic process. A more exothermic process releases enough heat to continue melting the ice around it. Dissolving requires energy to break apart the lattice structure of the solid salt, and then gives off energy as new bonds are formed with the water. Dissolving can be exothermic if stronger bonds are formed than are broken, or endothermic if stronger bonds are broken than are formed. In this experiment, it was predicted that anhydrous salts would have the most exothermic reactions with water and thus be the best ice-melts, because they do not have any bonds with water in the solid form to break in the dissolving process. Experimental This lab used a Styrofoam cup calorimeter (with a heat capacity of 55.8) and the Labworks program to obtain temperature data. The mass of the cup was measured first. 40mL of water was obtained in a graduated cylinder and added to the cup, and the cup and contents were weighed. The water temperature was measured for three minutes. In the meantime, approximately 2.0g of solute was set aside. After three minutes, the solute was added all at once Alison Poulson and the solution was stirred. The temperature was monitered for another 600 seconds. A graph of the temperature vs time was used to obtain the initial and final temperatures of the solution, and the solution was weighed to find the exact mass of the solute and of the solution. Using these data, the enthalpy of each reaction was calculated. Results The following graph of the reaction of CaCl2 is a sample of the graphs of temperature vs time obtained for each solute. In each case, a linear fit was made for the points before the time of mixing, and the linear equation was solved for the time of mixing to give the initial temperature. Another linear fit was made after the temperature steadied after mixing, and that equation was solved for the time of mixing to give the final temperature. Only the change in temperature is recorded for each solute in the table below. 25 y = -0.0015x + 23.468 R² = 0.9892 20 Series1 15 Series2 y = 0.002x + 16.166 R² = 0.9559 Series3 10 Linear (Series1) Linear (Series2) 5 0 0 200 400 600 800 1000 Alison Poulson Solute NaCl NaC2H3O2 CaCl2*2H2O C6H12O6 CaCl2 NaC2H3O2*3H2O 0.0323 0.0222 0.0127 0.0102 0.0163 0.0125 41.24 41.28 41.58 41.08 39.98 41.06 182.6 182.5 182.6 203.0 182.6 182.5 1.8854 1.5343 -0.6101 6.6635 -1.5123 Moles of Solute (mol) Mass of Solution (g) Time of Mixing (s) Change in Temperature -0.8854 (◦C) The data above was used to calculate qrxn using the following formulas: qrxn = -qsoln + -qcal qsoln = (mass solution)(specific heat capacity water)(change in temperature) qcal = (calorimeter constant)(change in temperature) The enthalpy change for the reaction was found using the following formula: ΔH = qrxn/molsolute/1000 The work is shown below for a sample, the reaction of CaCl2: qrxn = -(39.98g)(4.184J/g◦C)(6.6635◦C) + -(55.8J/◦C)(6.6635◦C) = -1486J ΔH = -1486J/0.0163mol/1000 = -91.2kJ/mol qrxn (J) NaCl NaC2H3O2 CaCl2*2H2O C6H12O6 CaCl2 NaC2H3O2*3H2O 202.2 -431 -353.1 -1486 343.8 138.7 Alison Poulson ΔH 6.26 -19.4 -27.8 13.6 -91.2 27.5 (kJ/mol) Discussion & Conclusions The results of the calculations match the experimental data, as reactions that resulted in a higher final temperature produced a negative enthalpy. The data seem to be accurate; there are no surprising or outlying results. A high-magnitude negative number for ΔH indicates a highly exothermic reaction. Therefore, according to these results, CaCl2 is the best ice melt. It releases 91.2kJ of energy for every mole used. As predicted, it releases less energy in its hydrated form – only 27.8kJ/mol – but this is still more energy than any other ice melt releases. NaC2H3O2 also follows the hypothesis. In its anhydrous form, it releases 19.4 kJ of energy per mole. Its hydrated form, by contrast, requires 27.5kJ/mol to melt. This is the most energy required by any of the solutes tested, so hydrated NaC2H3O2 is not appropriate for ice melt. Ice melt is often referred to as “salt,” NaCl, and blamed for damage to cars. However, NaCl is not a very efficient ice melt because it too requires energy to melt. Glucose is even less useful as an ice melt than NaCl. Overall, CaCl2 is the best choice for ice melt in terms of the heat it produces. However, cost or environmental considerations may play a role in determining which ice melt is finally chosen.