Chemical Reactions and Equations
advertisement

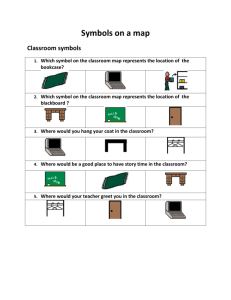
Chemical Reactions and Equations In this lab you will investigate several chemical reactions. In each case you will make observations and describe what you observe. You will make sure you have a word equation and a balanced chemical equation to describe what is happening. Chemical Equations A chemical equation shows what REACTANTS are present before a reaction and what PRODUCTS get made in the reaction. The simplest way to show this is in a word equation. Eg Hydrogen + oxygen Reactants water Products A symbol equation MUST be balanced. It shows what happens to individual atoms during the reaction. It is a rule of course that atoms do not change during reactions, they just combine together in different ways. That means you MUST show the same number of each type of atom present before and after the reaction. Balancing Hydrogen + oxygen water Sodium + Chlorine Sodium Chloride Methane + Oxygen carbon dioxide + water Reactions: You MUST wear safety glasses at all times. For each reaction: make sure you observe any temperature changes, gas being made (fizzing) and any colour changes. Is a solid being made? Is anything dissolving? Word Equation Magnesium + hydrochloric acid Symbol equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………………………… Word Equation Hydrogen + oxygen Symbol equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………………………… Word Equation Copper carbonate + hydrochloric acid Symbol equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………………………… Word Equation Copper Oxide + hydrochloric acid Symbol equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………………………… Word Equation sodium hydroxide + hydrochloric acid Symbol equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………………………… Word Equation potassium iodide + lead nitrate Symbol Equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………………………… Word Equation Magnesium + nitric acid Symbol equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………………………… Word Equation Copper carbonate + nitric acid Symbol equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………………………… Word Equation ethanoic acid + sodium carbonate Symbol equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………………………………………………………………………………… Word Equation sodium hydroxide + hydrochloric acid Symbol equation Observations ……………………………………………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………………………………………………