13_The Model so Far v2.1
advertisement

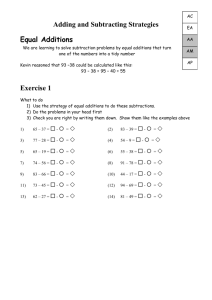
The Model so Far . . . Scientists perform experiments in order to learn about the natural world. They analyze the data they collect in order to explain various observations. Recall that explanations of scientific phenomena, known as theories, are most useful when they make reliable predictions. Oftentimes, scientists create models to describe, explain and predict phenomena within natural world. Throughout this chemistry course, we will create and refine a series of models to explain our observations. As we make new observations and analyze new data, we will find that we either need a new model or we need to add features to our current model in order for it to maintain its usefulness. You will find that the progression of the models created in our class mirrors the progression of the atomic theory from its roots in philosophy to its modern forms. The first model of the atom was proposed by Democritus and closely resembles the model we've used throughout Unit 1. As we progress through this course, you should use this packet to keep track of the features of our models of particle behavior as well as the scientists who first proposed such models. Democritus Features of the model include . . . Dalton Additions to the model include . . . Curie Additions to the model include . . . Modeling Chemistry 1 MSF v2.1 Thomson Additions to the model include . . . Milikin Additions to the model include . . . Rutherford Additions to the model include . . . Bohr Additions to the model include . . . Quantum Additions to the model include . . . Modeling Chemistry 2 MSF v2.1