The purpose of this lab was to determine an equilibrium
advertisement

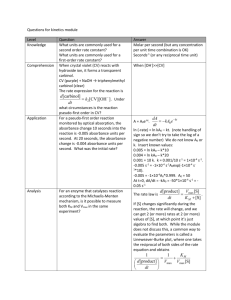
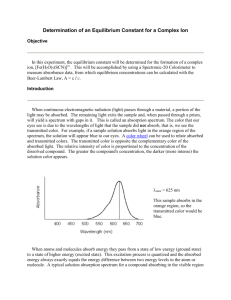
Kylie Case Period 6 Emma McKee Rebecca Smith Spectrophotometric Determination of an Equilibrium Constant Purpose: The purpose of this lab was to determine an equilibrium constant by observing and deriving calculations from the reaction between aqueous iron III nitrate and potassium thiocynate. Theory: A big part of this lab was knowing (and in the end proving) that absorbance is directly proportional to the concentration of a solution (Beer’s Law), and if Absorbance v. Concentration is graphed, a straight line will result. The regression line’s equation is used to determine the concentration of an unknown solution once the percent of T has been measured. The equation of Beer’s Law (A=abc) is an efficient way of putting the relationship between absorbance, the path length of the solution, and the concentration of the solution, into a numeric form, since it displays the ideal condition in which these components are proportional to the absorbance. Another important component in this lab was chemical equilibrium, which is the state in which the concentration of both the reactants and products yield no net change with time (This condition usually occurs when the forward reaction elapses at the same rate as the reverse reaction). A big part of chemical equilibrium is reaction constant K=[C]c [D]d / [A]a [B]b This equation can be calculated if the equilibrium concentration of each reactant and product in a reaction at the equilibrium in known. Procedure: In this lab, a laptop and colorimeter were set up as indicated by the instructor, followed by the group members obtaining the necessary testable chemicals. Next, five solution were prepared, which had varied amounts of KSCN and distilled water, with a constant of five mLs of Fe (NO3)3. Then the first solution was placed in a cuvette and the absorbance was tested using the colorimeter, and this data was plotted on a graph. This was then done for the remaining solutions, and using a compilation of the data, a best fit line was added to the graph, which was then printed. In part two, 5 mL of .2M Fe (NO3)3 were added to a 50 mL flask. The volumetric flask was filled with distilled water and then mixed thoroughly. The value of the absorbance was found, and the acquired data created a graph, was then interpolated to confirm the approximate value. In part three, five test tubes were used to create new solutions yielding various amounts of SCNand distilled water, and the volume of the .002M Fe(NO3)3 was kept constant at 3 mL. The colorimeter was used in order to find the absorbance of these solutions and collect data, and after all data was collected, the solutions were disposed of. Data: Table 1. Parts I and II: Beer’s Law Data Solution 1 .001 Absorbance 2 .255 3 .411 4 .567 5 .695 Unknown, Part II .513 Best-fit line equation for Part I standard solutions: _y=3521x-0.008505____________________ Temperature: _____23.1°C___________________ Concentration of Unknown: _____1.48 * 10-4__________________________ Table 2. Part III: Absorbance Data Test tube number 1 Absorbance Net Absorbance 0.000 2 0.125 0.125 3 0.198 0.198 4 0.246 0.246 5 0.307 0.307 Table 3. Initial Concentrations of SCN- and Fe3+ Test tube # [SCN-]i 2 4.00 * 10-4M 3 6.00*10-4M 4 8.00*10-4M 5 1.00*10-3M [Fe3+]i 6.00 *10-4M 6.00*10-4M 6.00*10-4 M 6.00*10-4M Table 4. Equilibrium concentration of FeSCN 2+ Test tube # [FeSCN2+]eq 2 3 4 5 3.79*10– Calculations: 1. Determine the initial concentration of SCN- and Fe3+ in the test tubes. Trial 2 Fe3+ SCN- Meq Veq= MiVi Meq Veq= MiVi Meq= MiVi/ Veq Meq= MiVi/ Veq Meq= (.0020)(3.00)/(.010) Meq= (.0020)(.0020)/(.010) Meq= 6.0 *10-4 Meq= 4.0*10-4 2. Use the net absorbance values in Part II and the best fit line equation in Part I to determine the [FeSCN2+] at equilibrium. Trial 2 Best-Fit Line Equation Net Absorbance Y= Graph: See attached graph Error Analysis: In this lab, as every other lab, there were multiple potential sources of error. Had everything been perfect, the Key values should have all been the same, which was not the case, though they were in close relation to eachother. A large source of error in this experiment could have been not making sure the test tubes were dry, as would have diluted the absorbance value which would in turn lower the value of Keq. Another source of error could have been temperature, since it was assumed that all solutions would remain at the same temperature, and body heat, or perhaps not being the temperature of the room at the time of reading, could have altered results and calculation. Conclusion: Throughout this lab, students determined an equilibrium constant by studying the reaction between aqueous iron III nitrate and potassium thiocynate, which was the purpose of the lab. Even though the results were not exactly as expected, the data helped the students derive values that were respectably close to the desired readings. The results tended toward the uppers 100’s lower 200’s, which were near what was expected. Overall, the students successfully carried out their task and continued to familiarize themselves with the lab equipment, as well as hopefully learn how to deal with the contingency of the temperature value fluctuating due to environmental influences.