Piperine (3
advertisement
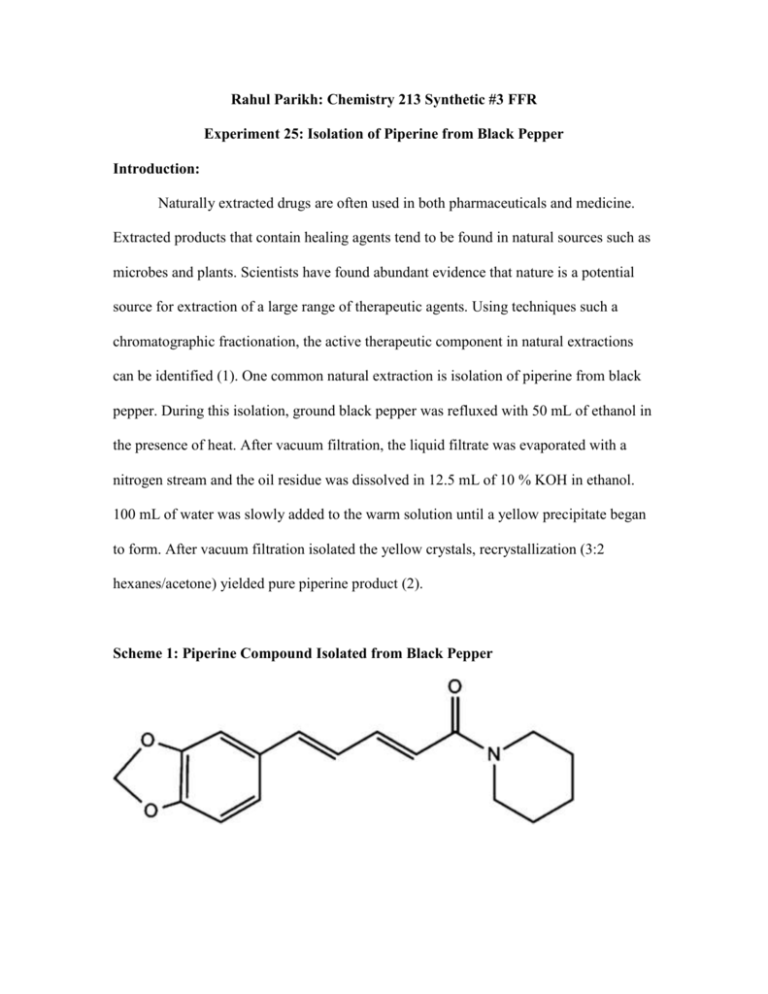
Rahul Parikh: Chemistry 213 Synthetic #3 FFR Experiment 25: Isolation of Piperine from Black Pepper Introduction: Naturally extracted drugs are often used in both pharmaceuticals and medicine. Extracted products that contain healing agents tend to be found in natural sources such as microbes and plants. Scientists have found abundant evidence that nature is a potential source for extraction of a large range of therapeutic agents. Using techniques such a chromatographic fractionation, the active therapeutic component in natural extractions can be identified (1). One common natural extraction is isolation of piperine from black pepper. During this isolation, ground black pepper was refluxed with 50 mL of ethanol in the presence of heat. After vacuum filtration, the liquid filtrate was evaporated with a nitrogen stream and the oil residue was dissolved in 12.5 mL of 10 % KOH in ethanol. 100 mL of water was slowly added to the warm solution until a yellow precipitate began to form. After vacuum filtration isolated the yellow crystals, recrystallization (3:2 hexanes/acetone) yielded pure piperine product (2). Scheme 1: Piperine Compound Isolated from Black Pepper Piperine, piperanine, and piperettine are common alkaloids that make up 5-9% of ground black pepper. These alkaloids, which are naturally occurring chemical compounds, can be extracted from black pepper in the presence of ethanol and KOH (2). Black pepper is composed of moisture, protein, sugar, amylose, and ash. It is one of the many peppers that aid in digestion, help balance a diet, and act as natural painkillers. Peppers also activate taste receptors associated with appetite. Piperine is an alcoholsoluble alkaloid responsible for monoclinic needles and the pungency of black pepper. It is composed of lignans, alkaloids, and oils. It has a variety of important biological properties, such as anti-antigenic and anti-cancerous properties, which prevent the formation of blood vessels that can potentially feed oxygen to cancerous tissue. Piperine also has anti-depression like activity, and exhibits cognitive enhancement in some cases. Lastly, piperine is been shown to stimulate anti-inflammatory responses, as well as skin pigmentation (3). The purpose of this experiment was to isolate piperine product from ground black pepper, using reflux with ethanol and dissolution in 10% KOH in ethanol. Precipitated piperine was purified via recrystallization (3:2 hexanes/acetone) to isolate the pure product. Using appearance, melting point range, as well as MS, IR, and NMR spectroscopy characterization methods, confirming the identity of piperine product became possible. Understanding how isolation of piperine from black pepper works allows chemists to carry out extraction of medicinal components from natural products on a much larger scale to provide treatment. Experimental: Piperine. Ground black pepper (12.5 g) was refluxed in the presence of ethanol (50 mL) for 90 minutes. After cooling the solution, vacuum filtration was used to isolate the liquid filtrate, which was then evaporated to concentrated oil with a stream of nitrogen. The oil residue was dissolved in 10% KOH in ethanol solution (12.5 mL). Water (100 mL) was slowly added to the warm solution until a yellow, foggy solution with precipitated crystals began to form. The solution mixture was refrigerated overnight after being tightly sealed. The precipitated piperine was collect using vacuum filtration and dried on the funnel for an hour. The piperine product was purified via recrystallization (3:2 hexanes/acetone) to yield a yellow, crystalline solid (1.108 g, 8.86 % recovery). 1H NMR (60MHz, CDCl3) δ (ppm) 1.62 (m, 6H), 3.56 (d, 4H), 5.976 (s, 2H), 7.0 (m, 6H); 1H NMR (400 MHz, CDCl3) δ (ppm) 1.62 (m, 6H), 3.56 (d, 4H), 5.976 (s, 2H), 6.4 (d, 1H), 6.78 (m, 3H), 6.8 (d, 1H), 6.9 (s, 1H), 7.4 (m, 1H); IR (ATR) υmax (cm-1) 1129.69, 1436.32, 1488.18, 1582.79, 1630.70, 2854.35, 2932.86; HRMS (ESI) m/z 285; MP (lit. value 130-131 °C) 129-131 °C. Results, Discussion, and Conclusions: Piperine was isolated from ground black pepper in the presence of ethanol, synthesizing yellow, crystalline solid piperine product after purification via recrystallization. Since piperine is a relatively polar compound, ground pepper was first refluxed with ethanol, allowing the piperine to be extracted from the ground pepper into the polar ethanol solution. Since the majority of ground pepper components (e.g. alkaloids, lignans) are less polar than piperine, their lack in solubility in ethanol prevented extraction of these components. After vacuum filtration, black pepper was removed while the ethanol solution containing piperine was evaporated with a stream of nitrogen and hot water bath, yielding viscous, concentrated, black oil. Use of water and 10% KOH in ethanol to dissolve the oil decreased piperine’s solubility in solution and assisted in precipitation of the piperine crystals after refrigeration. During this first part of the isolation, a foggy brownish yellow solution containing crystals was obtained. Using vacuum filtration, yellow precipitated piperine was isolated from the foggy solution. Finally, recrystallization (3:2 hexanes/acetone) was used to purify the precipitated piperine product. Nonpolar hexanes were more heavily favored in the recrystallization solvent mixture to allow the polar piperine product to precipitate out of solution after being cooled to room temperature and in a water/ice/salt bath. Purification via recrystallization helped identify the pure piperine product based on melting point and spectral data analysis (e.g. IR, MS, 60 and 400 MHz 1H NMR). While the literature recorded melting point range of piperine was 130-131 °C, the purified piperine melting point range was experimentally determined to be 129-131 °C (1). This consistent melting point range indicates that little to no impurities (e.g. ethanol, lignans, alkaloids) were present to potentially lower the product’s melting point. The isolation and purification of piperine was very successful with a recovery of 8.86 %, which falls with the 5-9% expected literature reported range (2). 60 MHz 1H NMR analysis of the isolated product (Figure 1) provided evidence that the product is in fact piperine. Due to lack of clarity, there are 4 chemically distinct hydrogens observed rather than the 11 chemically distinct hydrogens predicted. The 6H integral value at 1.62 ppm with an unclear splitting pattern represents the 6 very similar ethyl group hydrogens furthest from the amide functional group. An unclear splitting pattern at 3.56 ppm with a 4H integral value represents the 4 very similar ethyl group hydrogens closest to the amide group on piperine. Due to inductive effect from the amide group, these ethyl hydrogens are more downfield. Likewise, oxygen’s inductive effect on the carbon of an O-C-O group means that the singlet with a 2H integral value downfield at 5.97 ppm represents the 2 ethyl group hydrogens adjacent to the oxygens. Finally, 7 distinct H’s with unclear splitting patterns were clustered in the vinylic and aromatic regions between 6.3 ppm – 7.27 ppm. The 6H integral value around 6.78 ppm indicates that the 60 MHz 1H NMR did not pick up one of the vinylic hydrogens. Since piperettine has 2 additional chemically distinct vinylic H’s between 4.5 ppm – 6.5 ppm and piperanine has 2 additional chemically distinct ethyl H’s between 1.2 ppm- 1.4 ppm that aren’t present in the NMR, other alkaloids can be eliminated as a possibility for the isolated product. 400 MHz 1H NMR analysis of the isolated product (Figure 2) provided clearer splitting patterns and evidence to confirm the presence of piperine. There were 8 chemically distinct hydrogen’s are observed. The multiplet with a 6H integral value at 1.644 ppm represents the 6 very similar ethyl group hydrogens furthest from the amide functional group. The doublet at 3.564 ppm with a 4H integral value represents the 4 very similar ethyl group hydrogens closest to the amide group on piperine. Inductive effect from the amide group causes these ethyl hydrogens to be more downfield. Oxygen’s inductive effect on the carbon of an O-C-O group indicates that the downfield singlet with a 2H integral value at 5.976 ppm represents the 2 ethyl group hydrogens adjacent to the oxygens. The 400 MHz 1H NMR provided much more clarity on the aromatic and vinylic hydrogens in the region between 6.4 ppm – 7.4 ppm. Due to the amide’s inductive effect, along with the conjugated system present in piperine, the 1H multiplet at 7.4 ppm represents the downfield and deshielded vinylic hydrogen two carbons left of the carbonyl. Likewise, the same principles indicate that the upfield and shielded 1H at 6.4 ppm represents the vinylic hydrogen on the adjacent left of the carbonyl. The 3H multiplet at 6.78 ppm represent the aromatic hydrogen furthest from the O-C-O group, along with the two vinylic hydrogens closest to the aromatic ring. Finally, the 1H singlet at 6.9 ppm and the 1H doublet at 6.8 ppm represent the top and bottom aromatic hydrogens respectively. Other alkaloids such as piperettine and piperanine have 2 additional chemically distinct vinylic H’s between 4.5 ppm – 6.5 ppm or 2 additional chemically distinct ethyl H’s between 1.2 ppm- 1.4 ppm, respectively, that aren’t observed in the NMR. This observation helped rule out these other two alkaloids as possible isolated product. Infrared Spectroscopy analysis of the isolated product (Figure 3) provided inconclusive evidence to distinguish the isolated product from other alkaloids with identical functional groups such as piperanine and piperettine. Instead, it helped confirm the structural identity of piperine after NMR identification. IR spectroscopy was used to identify and confirm the presence of piperine’s functional groups. The O-C-O stretch was observed at 1129.69 cm-1 while the aliphatic C-H stretch of piperine was observed at 2854.35 74 cm-1. Although aromatic C-H stretch was not located, it is likely to be present in the overlapping region adjacent to the alkene C-H stretch observed at 2932.86 cm-1. A scissor bend –CH2 stretch was observed at 1436.32 cm-1, and an aromatic C=C stretch was located at 1488.18 cm-1. Finally, a tertiary amide stretch was observed at 1630.7 cm-1 and an alkene C=C stretch was observed at 1582.79 cm-1. Mass spectroscopy produced conclusive evidence that piperine was the isolated product. According to the MS data (Figure 4), piperine’s molecule weight is 285 atomic mass units as indicated by the parent peak. The base peak with the largest ion fragment cleaves the nitrogen ring to leave behind an acylium ion with a weight of 201 amu. Upon fragmentation of the bond between the carbonyl carbon and C=C, two other M/Z fragments are observed. The acylium ion and its corresponding vinylic ion fragment have molecular weights of 115 amu and 173 amu respectively. The other two alkaloids in black pepper, piperanine and piperettine, both have molecular weights over 285 amu by 2 amu and 26 amu respectively. Therefore, the isolated product cannot be either of these two other alkaloids. Upon completion of the isolation, piperine was successfully isolated from black pepper since the 8.86 % recovery falls with the 5-9% literature reported range (2). The purified product was a yellow, crystalline solid. Melting point, NMR, IR, and MS spectral data of the product collectively confirmed that the isolated alkaloid was indeed piperine. For future advancements, one can explore alternative techniques to remove components of different polarities from black pepper, such as column chromatography. References: 1. Hong-Fang Ji, Natural products and drug discovery, NCBI EMBO Report, 2009; 10, 194–200. 2. Epstein, W. W.; Netz, D. F.; Seidel, J. L. J. Chem. Ed. 1993, 70, 598-599 3. Ind. Eng. Chem. Res.2002, 41, 2521-2528 4. J. Chem. Ed. 1993, 70, 598 5. MSDS, Piperine, No. 64622, Sigma-Aldrich, 11/28/14 http://www.sigmaaldrich.com/MSDS/9926579









