Wordfile
advertisement
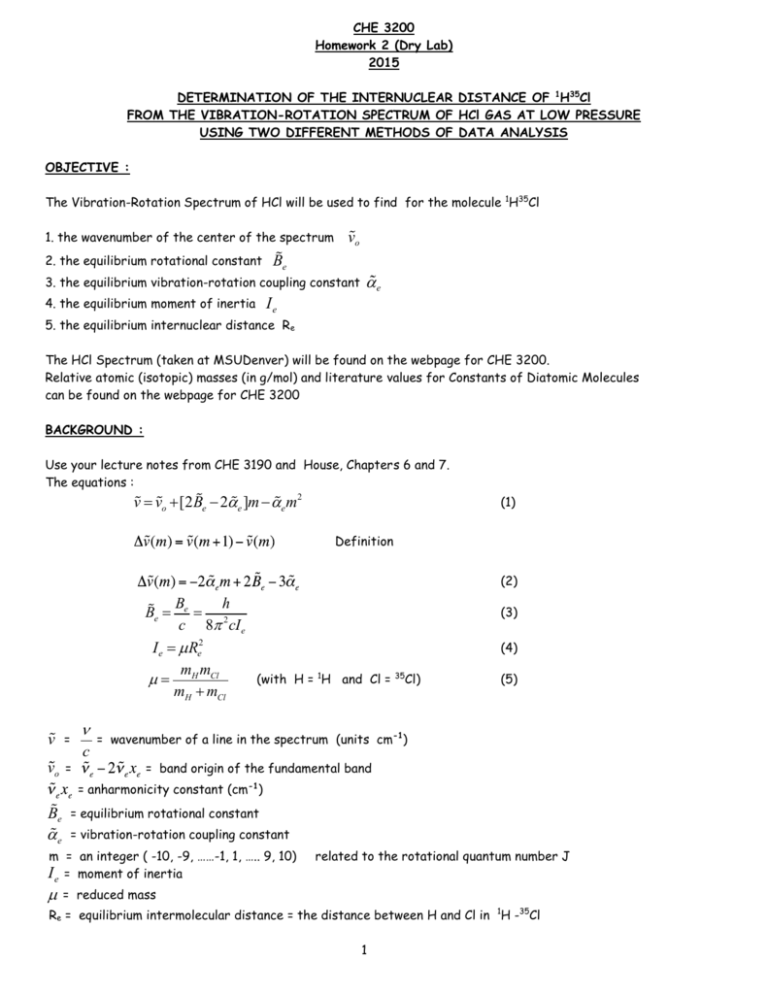
CHE 3200 Homework 2 (Dry Lab) 2015 DETERMINATION OF THE INTERNUCLEAR DISTANCE OF 1H35Cl FROM THE VIBRATION-ROTATION SPECTRUM OF HCl GAS AT LOW PRESSURE USING TWO DIFFERENT METHODS OF DATA ANALYSIS OBJECTIVE : The Vibration-Rotation Spectrum of HCl will be used to find for the molecule 1H35Cl 1. the wavenumber of the center of the spectrum 2. the equilibrium rotational constant vo Be 3. the equilibrium vibration-rotation coupling constant 4. the equilibrium moment of inertia ae Ie 5. the equilibrium internuclear distance Re The HCl Spectrum (taken at MSUDenver) will be found on the webpage for CHE 3200. Relative atomic (isotopic) masses (in g/mol) and literature values for Constants of Diatomic Molecules can be found on the webpage for CHE 3200 BACKGROUND : Use your lecture notes from CHE 3190 and House, Chapters 6 and 7. The equations : v = vo +[2Be - 2ae ]m - ae m2 (1) Definition (2) Be h = 2 c 8p cI e I e = m Re2 m m (with H = 1H and Cl = 35Cl) m = H Cl mH + mCl Be = v = vo = n c (3) (4) (5) = wavenumber of a line in the spectrum (units cm-1) = band origin of the fundamental band = anharmonicity constant (cm-1) Be ae = equilibrium rotational constant = vibration-rotation coupling constant m = an integer ( -10, -9, ……-1, 1, ….. 9, 10) I e = moment of inertia m related to the rotational quantum number J = reduced mass Re = equilibrium intermolecular distance = the distance between H and Cl in 1H -35Cl 1 DATA : Use the data found on the webpage for this class. For each of the 20 lines of 1H-35Cl record the wavenumber in cm-1 Pay attention to the m value for each line. The 1H-35Cl lines are the more intense lines. The weaker lines are from 1H-37Cl (in natural abundance). CALCULATIONS : METHOD 1 : (SOLVER) using equation 1 You will use the EXCEL Tool SOLVER (which uses the ‘Principle of Least Squares’ to fit an equation with up to 200 independent variables to experimental data). You will use one equation with three unknowns (equation 1). The data are the wavenumbers of 20 lines of the vibration-rotation spectrum of 1H35Cl gas. vo , Be The three unknowns are : and ae Open a new Excel spreadsheet and enter the first two columns using data from the HCl Rotation-Vibration Spectrum (m in column A, wavenumber v in column B) A B C D 1 CHE 3200 Homework 2 Exercise using SOLVER 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 di2 m -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 1 2 3 4 5 6 7 8 9 10 vo = Be = ae = 2 In cell B26 enter a reasonable value for In cell B28 enter a reasonable value for In cell B30 enter a value for In cell C4 calculate v vo (the wavenumber of the center of the spectrum , the ‘band-origin’) Be (this is half of the separation of the lines in cm-1) a e ( a value between 0 and 5 works well) using equation (1) and the values for vo , Be , and a e you entered ($B$26, $B$28, and $B$30). These are the three variables which will be found by SOLVER. Copy down. In cell D4 calculate d2 =(B4-C4)2 . Copy down. In cell D24calculate SUM of all di2 Leave cell D24 selected and call up Solver (Tools Solver). The following window will appear: Enter : Set Objective : $D$24 (if it is not already entered) To : Min By changing variable cells : $B$26,$B$28,$B$30 Click on : Solve For more details on SOLVER see the handout CHE 3200 PARTICLE IN THE BOX (2015) SOLVER will return optimized values for Be a e in cells B26 , B28, B30 (it will take a little time for SOLVER to do the iterations). Use the optimized value of Be (cell B28) to calculate the moment of inertia Ie and the intermolecular distance Re of 1H35Cl using equations (3), (4), and (5) . Show the calculations. Pay attention to units. 3 METHOD 2 : (LINEST) using equation 2 For more details on how to use LINEST refer to the handout : CHE 3200 Linear Least Squares Analysis using LINEST 2015 Open a new Excel spreadsheet and enter m values as shown in the first column. Enter values in the second column. These values are calculated from the measured values which you have in your first spreadsheet using the definition Example : Note : there is no and no because there is no m -10 -9 -8 -7 -6 -5 -4 -3 -2 1 2 3 4 5 6 7 8 9 25.73 Highlight a 2column x 3row matrix and perform a LINEST calculation . Use the values for the slope and the intercept and equation 2 of this handout to calculate ae and Be Unfortunately the m in equation 2 is not the same as the m usually used for the slope. If equation 2 is a straight line then the slope is -2 a e and the y-intercept is Calculate a e first from the slope and then use this a e to calculate from the intercept. Include your calculations. ERROR : Derive the equations for the error in a e and the error in Be using the error in the slope and the error in the y-intercept. Derive the equations for the error Ie and the error in Re using equation (3) and (4) and assuming that only Be has an error. Calculate the error in a e and Be from the errors in the slope and intercept from you LINEST analysis. Calculate the error in Ie and the error in Re using the error in 4 Be Include your calculations. REPORT : Report your values of : (a) (b) Be (c) ae (d) Ie m (e) (f) Re from your SOLVER analysis (without errors). Report your values of : (a) Be (b) ae (c) Ie (d) Re from your LINEST analysis with errors Report literature values for , , Be , ae , Ie , Re for 1H35Cl Calculate the band origin from the literature values . Include your Excel Spreadsheets using SOLVER and LINEST Suggestion : It is always nice to organise the results in a table Example : SOLVER LINEST ------------ ERROR ------------ ------------ ------------ ------------ ------------ ------------ ------------ Ie/units Re/units 5 Literature Reference








