New materials for hydrogen storage
advertisement

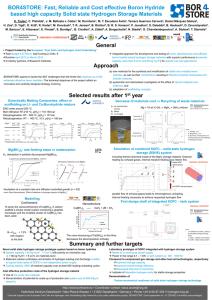
SMEC2015, conference at Miami - Western Caribbean. Hydrogen Storage, Production & Fuel Cell, March 8 - 15, 2015 NEW MATERIALS FOR HYDROGEN STORAGE Kasper Møller, Lars Jepsen, Mark Paskevicius, Torben R. Jensen * Center for Materials Crystallography (CMC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Denmark (trj@chem.au.dk). Hydrogen is recognized as a potential and extremely interesting energy carrier, which can facilitate efficient utilization of unevenly distributed renewable energy. Here we report an overview of our recent results within new materials for hydrogen storage: (i) synthesis of novel metal borohydrides and studies of their properties, (ii) tailoring materials properties by formation of eutectic melting systems or ammonia containing compounds, and (iii) in situ powder X-ray diffraction for studies of hydrogen release and uptake reactions. We conclude that the chemistry of hydrides is very divers, towards multi-functional materials, including ionconductors for batteries, methanesation of carbondioxide etc. Metal borohydrides Hydrogen has the highest gravimetric energy density of all known substances and is attractive for future storage of renewable energy [1]. We have recently developed new synthesis strategies combining mechanochemical and solvent based methods for synthesis of new hydrides. The metal borohydrides MM’BH4, typically contain an alkali metal, M, and a di- or tri-positive cation, M’. Apparently, the structural complexity increase with the increasing size of the alkali metal and also the tendency to form mixed borohydride-halide compounds and ternary chlorides [1-4]. A fascinating structural chemistry is discovered within metal borohydrides, e.g. interpenetrated ‘MOF-like’ networks or zeolite-type structures with up to 30% ‘empty’ space in the porous structures of M(BH4)2, M = Mg or Mn, which additionally can physisorp molecular hydrogen, e.g. Mg(BH4)2~2H2, m = ~20 wt% H2 [5,6]. Another polymorph has extremely dense packing of hydrogen, Mg(BH4)2, V = 147 g H2/L. Recently, we stabilized NH4BH4 with extreme hydrogen density, 24.5 wt% H2, as NH4Ca(BH4)3 (15.7 wt% H2) with higher decomposition temperature, Tdec = 97 C [7]. We have also developed new synthesis methods and prepared series of M(BH4)m∙nNH3 (M = Mn, Y, Gd, Dy etc; 7 n 1.). The n/m ratio can be used to tune the composition of the released gas in favour of hydrogen. Ion conductors for batteries Recently, a series of new fast Li-ion conductors, LiM(BH4)3Cl, M = La, Ce, Gd, Pr or Nd, which simultaneously store significant amounts of hydrogen was discovered. The conductivity is due to unoccupied cation position around the large cubane like composite anions [8]. Multi-functionality A series of 30 new complex hydride perovskite-type materials and new synthesis protocols involving rare earth elements was recently presented [7]. Photophysical, electronic and hydrogen storage properties was discovered along with trends in structural behaviour. In this view, homopolar hydridic di-hydrogen contacts arise as a potential tool to tailor crystal symmetries, hence merging concepts of molecular chemistry with ceramic-like host lattices. Furthermore, anion-mixing provides a link to the known ABX3 halides. Some metal hydrides are found to react with carbon dioxide and provide a new approach for direct metahanisation of CO2. In this talk, we will also illustrate that in situ powder X-ray diffraction is a unique, sensitive and informative technique for probing gas-solid reactions. References [1] M. B. Ley, et al, Mater. Today, 2014, 17, 122. [2] Rude, et al, Physica Status Solidi 2011, 208 1754. [3] L. Jepsen, et al, Mater. Today, 2014, 17, 129. [4] J. Huot, et al. Prog. Mater. Sci. 2013, 58, 30. [5] E. Roedern, et al, J. Phys. Chem. C, 2014, 118, 23567. [6] Filinchuk, et al, Angew. Chem. Int. Ed. 2011, 50, 11162. [7] P. Schouwink, Nature Comm. 2014, DOI: 10.1038/ ncomms6706. [8] Ley et al. Chem. Mater. 2012, 24, 1654.
![DIRECT SYNTHESIS OF Li[BH4] FROM THE ELEMENTS](http://s3.studylib.net/store/data/006749722_1-3acc3b7e04414ccf23cb4364d250a1e7-300x300.png)








![Synthesis and crystal structure of [UO2(BH4)2(hmpa)2], a new](http://s3.studylib.net/store/data/007682111_2-b412267f6dd41ac7b7d7e9c7bb2cc85b-300x300.png)