Unit 4 Study Guide Answers
advertisement
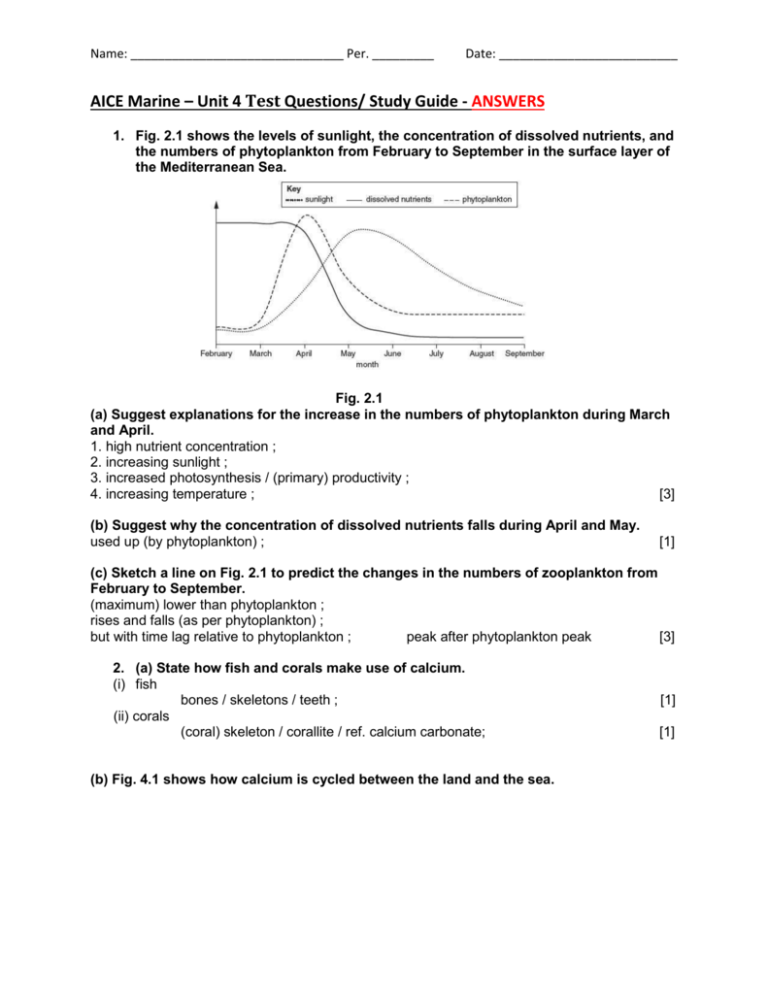
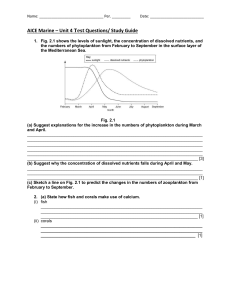
Name: _______________________________ Per. _________ Date: __________________________ AICE Marine – Unit 4 Test Questions/ Study Guide - ANSWERS 1. Fig. 2.1 shows the levels of sunlight, the concentration of dissolved nutrients, and the numbers of phytoplankton from February to September in the surface layer of the Mediterranean Sea. Fig. 2.1 (a) Suggest explanations for the increase in the numbers of phytoplankton during March and April. 1. high nutrient concentration ; 2. increasing sunlight ; 3. increased photosynthesis / (primary) productivity ; 4. increasing temperature ; [3] (b) Suggest why the concentration of dissolved nutrients falls during April and May. used up (by phytoplankton) ; [1] (c) Sketch a line on Fig. 2.1 to predict the changes in the numbers of zooplankton from February to September. (maximum) lower than phytoplankton ; rises and falls (as per phytoplankton) ; but with time lag relative to phytoplankton ; peak after phytoplankton peak [3] 2. (a) State how fish and corals make use of calcium. (i) fish bones / skeletons / teeth ; (ii) corals (coral) skeleton / corallite / ref. calcium carbonate; (b) Fig. 4.1 shows how calcium is cycled between the land and the sea. [1] [1] Name: _______________________________ Per. _________ Date: __________________________ Fig. 4.1 Name processes D, F and G. D harvesting / fishing / feeding / eating / consuming ; F upwelling ; G ref. to (part of) food chains ; A uptake / intake / consuming / feeding (i) [3] (ii) Describe how calcium on land becomes a nutrient in the surface layer of the sea. 1. present in rocks / soils / fertilisers ; 2. dissolve in (fresh) water ; A ref. to leaching 3. (carried to sea) in rivers / streams / runoff ; [3] (iii) Describe process E. 1. death of organisms / excretion / egestion ; 2. sinks / AW (to sea bed) ; 3. decomposition / decay / ref. decomposers ; 4. ref. action of bacteria ; [3] 3. (a) State one biological use of each of the following nutrients in marine ecosystems. (i) nitrogen (named) proteins ; A other N-containing organic substances, e.g. amino acids, DNA [1] (ii) magnesium chlorophyll ; A bone / shell [1] (iii) phosphorus DNA / bone ; A other P-containing organic substances, e.g. phospholipids, ATP [1] (b) Suggest why an increase in the concentration of carbon dioxide in the atmosphere could result in an increase in the productivity of consumers in a marine ecosystem. 1. (increased) dissolution (of carbon dioxide) ; 2. (increased) availability of carbon dioxide / hydrogencarbonate to producers / named example; A CO2 no longer a limiting factor to producers 3. (increase in) photosynthesis ; 4. (more) organic substances produced ; A named example, e.g. glucose 5. increased productivity of producers ; Name: _______________________________ Per. _________ Date: __________________________ 6. more food / organic substances available to consumers / next trophic level / AW ; A more energy / biomass 7. biomass of consumers increases ; [5] (c) Discuss the reasons for the variability of the concentration of dissolved oxygen in sea water. 1. turbulence / wave action ; A water movement at surface / A mixes oxygen with water 2. helps (atmospheric) oxygen to dissolve ; 3. photosynthesis ; 4. produces oxygen / increases oxygen ; A algae / producers produce oxygen 5. respiration ; 6. uses oxygen / reduces oxygen ; A consumers use up oxygen 7. temperature ; 8. reference to reduced solubility as temperature increases / ORA ; 9. depth ; 10. (dissolved) oxygen decreases (as depth increases) / ORA ; [7] 4. (a) Fig. 7.1 shows the changes in the concentration of carbon dioxide in the atmosphere, the concentration of carbon dioxide in sea water and the pH of sea water from 1990 to 2010. Fig. 7.1 (i) Describe the relationship between the concentration of carbon dioxide in the atmosphere and the concentration of carbon dioxide in sea water shown in Fig. 7.1. Suggest an explanation for this relationship. relationship as carbon dioxide (concentration) in atmosphere increases, carbon dioxide (concentration) in sea water increases / both increase / ora ; explanation any 2 of: Name: _______________________________ Per. _________ Date: __________________________ carbon dioxide in air dissolves in sea water / forms a solution / dissolution / diffuses into ; carbon dioxide not very soluble in sea water ; carbon dioxide from sea water enters air + carbon dioxide from air enters sea ; reference to equilibrium ; [3] (ii) State the relationship between the concentration of carbon dioxide in sea water and the pH of sea water shown in Fig. 7.1. Suggest an explanation for this relationship. relationship as (concentration of) carbon dioxide in sea water increases, pH falls / ora ; explanation + any 2 of: more carbon dioxide dissolves in sea water / forms a solution / dissolution / diffuses into ; forms carbonic acid / bicarbonate ions ; ref. low pH is (more) acidic ; [3] (b) Calcium is present in rocks on land. Explain how calcium from these rocks can become part of the sediment at the bottom of the ocean. any 4 of: ref. to erosion / rain / precipitation / fresh water / leaching / runoff ; drained into ocean / sea ; enters food chain / description, e.g. taken up / used by algae / phytoplankton / organisms ; used by corals to form skeleton ; ref. forms bones / shells ; reference to calcium carbonate ; reference to death / decay of organisms ; dead organisms / waste products / detritus / faeces sinks / moves to sea bed ; [4] 5. Carbon is an element found in many different compounds in living organisms and in the environment. (a) Name one compound found in living organisms that contains carbon. any 1 named organic compound ; [1] (b) Fig. 4.1 shows some of the ways in which carbon can move between the atmosphere, oceans and sediment at the bottom of the ocean. The numbers are gigatonnes of carbon per year. Name: _______________________________ Per. _________ Date: __________________________ Fig. 4.1 (i) Name the form in which carbon enters the ocean from the atmosphere. carbon dioxide ; [1] (ii) Suggest one human activity which contributes to the 2 gigatonnes of carbon added to the surface ocean each year. burning / combustion of fossil fuels ; [1] (iii) Name process A shown in Fig. 4.1. upwelling ; [1] (iv) Suggest how the 0.1 gigatonnes of carbon are released from the ocean sediments. dissolving of shells / rocks / erosion / weathering / owtte ; [1] (v) The amount of carbon in the surface ocean stays approximately the same each year. Explain why. amount taken in for photosynthesis equals amount given out by respiration / owtte ; reference to figures – 90 in, 90 out ; amount from upwelling and death / decay is the same ; reference to figures – 37 and 37 ; [3] (vi) The amount of carbon added to the surface ocean each year by human activities is increasing. Suggest and explain one consequence of this increase. acidification of sea water / decrease in pH / owtte ; reference to any named effect, e.g. dissolves skeletons of corals ; reference to increase in productivity / increased rate of photosynthesis ; [2] Name: _______________________________ Per. _________ Date: __________________________ 6. (a) Runoff is important in replenishing the reservoir of dissolved nutrients in the sea. (i) Describe what is meant by the term runoff. draining away / flow of water from the land (surface); I leaching carrying nutrients / minerals / nitrates / phosphates / other named / pesticides / herbicides / sewage / oil; into sea / river / watercourse / lake; caused by rain / snow melt / irrigation water / owtte; [3] (ii) Suggest how runoff can be harmful to marine organisms. any 3 of: example of pollutant / excess nutrients / excess nitrates / phosphates / other named / pesticides / herbicides / insecticides / sewage / oil / heavy metals / named example; absorbed / taken up by marine organisms; passed through food chain; A – bioaccumulation toxins / poisons toxic / death; algal bloom; carries sediments; block light to producers / corals; [3] (iii) Suggest how runoff can be beneficial to marine organisms. carries nutrients / minerals / e.g. calcium* / other named e.g. / fertiliser; * linked mark increased photosynthesis / productivity / growth / reproduction / for shells* / bone* / skeletons*; [2] (b) The mean concentration of calcium in sea water is 0.41 parts per thousand. Suggest why this concentration remains approximately constant. any 4 of: removed when used to make coral / shells / skeletons / bones; removed by sinking to sea bed; removed by being incorporated into limestone rocks; ; added in runoff; added by upwellings (from deep water); added when organisms die; [4] 7. Various nutrients, including carbon, are cycled in marine ecosystems. Fig. 2.1 below shows part of the carbon cycle in the marine ecosystem in one year. The figures are in 1012 kg of carbon per year. Name: _______________________________ Per. _________ Date: __________________________ (a) Explain what is happening in process A shown in Fig. 2.1. carbon dioxide ; [accept CO2] dissolves / reference to dissolution ; reference to formation of HCO3 / H2CO3 ; [accept words] [2] (b) Using the information in Fig. 2.1, calculate the annual change in the carbon in surface water. Show your working. (92.5 + 40 + 36) – (90 + 38 + 40) or 168.5 – 168 ; = 0.5 ; × 1012 kg (per year) ; [accept 5 × 1011 kg] [correct answer with units gains 3 marks] [could award the units mark if calculation is incorrect] [3] (c) With reference to Fig. 2.1, explain why an increase in carbon in the atmosphere could lead to an increase in the productivity of organisms in the surface water of an ocean. more carbon (dioxide) dissolves ; (therefore) the concentration in (surface) water increases / eq ; more carbon (dioxide) available for photosynthesis ; of producers ; [accept equivalents such as ‘phytoplankton’, ‘aquatic plants’, etc] Name: _______________________________ Per. _________ Date: __________________________ more food available to higher trophic levels / consumers / eq ; [accept references to an increase in biomass / increase in primary production] [3] 8. (a) Complete Table 3.1 to show one biological use for each nutrient. [3] (b) Fig. 3.1 shows how nutrients are cycled in the sea. (i) Name the processes labelled A and B. A uptake / absorption by plants / organisms / owtte; B (nutrients / detritus) sinking / used in reef building / owtte; [2] Name: _______________________________ Per. _________ Date: __________________________ (ii) Describe what is meant by upwelling. movement of deep / bottom (ocean) water to (ocean) surface; + any 2 of; reference nutrients; reference to movement of surface water / currents / wind; reference to deflection of deep ocean currents; [3] (iii) Identify two processes, shown in Fig. 3.1, that are affected by human activities. For each process, briefly describe how humans are involved. process 1: fishing; human involvement reference to one positive effect / negative effect; process 2: run off / dissolution; human involvement reference to agriculture / chemicals / fertilisers / pollutants washed into sea / dissolve in water; [4] 9. (a) Describe the biological roles of magnesium, calcium and phosphorus in marine ecosystems. magnesium for: (synthesis of) chlorophyll; calcium for: bones; corals; shells; formation of carbonates/corallite; phosphorus for: DNA; ATP; phospholipids; bone; formation of calcium phosphate/apatite; [6] (b) Organisms living in the surface layer of an ocean take up nutrients. Describe the ways in which these nutrients may then be lost from this surface layer. credit references to: dead organisms/organisms die/detritus; faeces; sinking to sea floor; ref to (slow) decomposition on sea bed; (because) cold/lack of oxygen; Name: _______________________________ Per. _________ Date: __________________________ incorporation into reefs; harvesting/eq; [4] (c) Explain how the nutrients in the surface layer of an ocean are replenished. credit references to: upwelling; due to currents/explanation of upwelling/eq; further details (e.g. of inorganic nutrients released by decomposition); leaching/run off (from land); e.g. phosphates or nitrates; dissolving of atmospheric gases; carbon dioxide; [5] 10. Fig. 7.1 shows the phosphorus cycle on land and in the sea. Fig. 7.1 (a) Describe how phosphorus in soil becomes part of the tissues of marine fish. 3 of: dissolves in soil water; run off into rivers into sea; taken in/used in photosynthesis/used in protein synthesis by phytoplankton/algae; passes along food chain to fish; [3] Name: _______________________________ Per. _________ Date: __________________________ (b) Fig. 7.2 shows the winter concentration of phosphate in the surface water of the Baltic sea from 1970 to 2000. Fig. 7.2 (i) Table 7.1 shows the amounts of phosphate-containing fertiliser used from 1970 to 2000. Plot this data on the graph using an appropriate scale on the right hand axis. Name: _______________________________ Per. _________ 15 correct plots;;; correct line joining all plots; Date: __________________________ – 1 each incorrect [4] (ii) Calculate the rate of increase in the phosphate concentration from 1970 to1982. Show your working. 0.12 μmol/dm3 per year; [2] (iii) After 1974 the use of fertiliser fell. Explain why the concentration of phosphate in the sea continued to rise. some fertiliser remains in soil and run off continues; [1] (iv) Suggest a reason for the large increase in phosphate concentration from 1992 to 1994. year of above normal rainfall carries phosphates from soil to sea; [1]