Regents Chemistry Course Outline
advertisement

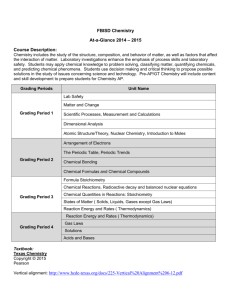
Regents Chemistry Course Syllabus Instructor: Brian Gallagher Email: Brian.Gallagher@rcsdk12.org Room: A253 ex. 2530 Course Description: This course is designed to introduce students to the fundamental concepts in chemistry, the science that studies the materials of the universe and the changes that these materials undergo. Learning chemistry can be very rewarding and exciting because it provides explanations for real world phenomena. The goal of his course is to provide students with the knowledge and opportunity to apply scientific thinking and relate concepts, principles, and theories in chemistry to their everyday lives. Upon completion of this course, students are expected to have developed the following: A deep understanding of fundamental concepts in chemistry Critical thinking skills Proficiency in chemistry laboratory procedures Mastery in solving math-based chemistry problems Grading Policies: All grades will be entered into the District’s online grading system, Power Teacher. Parents can view their students’ grades at https://parentconnect.rcsdk12.org/. Grades for this course will be broken down into weighted categories: Classwork Projects/Labs Tests/Quizzes Homework 20% 20% 50% 10% There will be a learning log that records daily topics and the question of the day as well as summary of the lesson. This is to be completed daily and is checked and/or collected on Fridays. (10 Points) Classwork will be based on activities in class and on participation in classroom discussions. Notebooks will be checked 4 to 5 times a marking period for a grade. (10-20 points) Quizzes will be given every Friday on the material we were covering. (10 points) Tests will be announced will mostly cover a broader topic. They will be regents styled. (50-60 points) Homework will be assigned throughout the week and written assignments are collected and graded. (5-10 Points) Late and incomplete assignments can be handed in late and 2 point will be subtracted for each day it is late. DUE TO THE SHIFT IN MARKING PERIODS THE POINT VALUES MIGHT BE ADJUSTED Lab Policies: Students must satisfactorily complete 1,200 minutes of lab exercises to be eligible to take the Regents exam The 1,200 minute Lab Requirement must be met by the first week of June 2015. Labs are due before the next lab period There will be no make-up opportunity for students who were illegally absent or are asked to leave the room by the lab teacher for unacceptable behavior or a safety violation. Failure to hand in labs will result in a grade of 0 on the Regents exam and failure of the course. Lab Grading Policies: Quality: All labs should be neat, organized, and legible. Follow all appropriate conditions for scientific notation, significant figures and problem solving. Do not "fudge" data to arrive at what you think is a correct answer. Grading: Completed labs will be graded on a scale of 1 to 10. If all basic lab requirements above are completed, a grade of 7 out of 10 will be awarded. Deductions: 1 point will be deducted for each basic lab requirement that is not correctly completed. Examples: Missing Conclusion -1 Missing Graph -1 Diagram has no labels -1 Additions: 1 point will be added each time a basic lab requirement is exceeded. Examples: Lab is typed +1 Exceptionally well-written conclusion +1 Lab is turned in on the next school day +1 No grade higher than 10 out of 10 can be awarded. Required Materials: Binder with loose leaf paper for notes and to organize handouts. Pens and pencils, as well as colored pencils Textbook, and review books provided by the library SCIENTIFIC calculator Classroom Expectations: Be on time, Be prepared, Be engaged. o Early is on time! On time is late! Cell phones and all Electronics are prohibited in ALL classrooms. o To include but not limited to: ear buds, headphones, IPODS. The Dress code will be strictly enforced. o To include but not limited to: Hats, hoodies and bandanas as well as what is prescribed by the school handbook and posters. Disrespect/Disruption to the educational process is prohibited. o To include but not limited to: bullying, profanity, food consumption and personal grooming. NON-NEGOTIABLES – Immediate removal from classroom o To include but not limited to: Fighting; both physical and verbal, vandalism, theft, chronic disruption, and suspicion of being under the influence of illegal substances. CONSEQUENCES: To include but not limited to: o Verbal/non-verbal warning/contact log, Move the student’s seat, Parent contact, Lunch detention, After school detention, Conference with administrator Regents Chemistry Course Outline 1. Scientific Method & Measurement Prefixes, Scientific Notation, Significant Figures, Percent Error, and Graphing 2. Matter & Energy States of Matter, Matter vs. Energy, Mixtures vs. Pure Substances 3. Atoms, Ions, & Isotopes Protons, Neutrons, Electrons (charge and location), Ions (both positive and negative), Isotopes, Electron configurations (ground state, excited state, bright-line spectra, valence electrons) 4. The Periodic Table Metals, Nonmetals, Transition Metals, Names of Groups and their properties (periods vs. groups), Trends (valence electrons, principle energy levels, atomic radii, ionic radii, shielding, ionization energy, electronegativity, 7 H Club) 5. Bonding Intramolecular vs. Intermolecular forces of attraction, Energy and Bonds, Lewis Dot Structures (Ionic and Covalent), Octet Rule, Ionic Bonds (Properties, identifying, drawing), Metallic bonds (properties and identifying), Covalent bonds (Polar vs. Nonpolar, Single, Double, Triple Bonds, Molecular Shape and Polarity, Macromolecules/Network Solids) 6a. Chemical Formulas & Equations Coefficient, subscript, formula, Molecular vs. Empirical Formula, Ions, Hydrates, Anhydrous Salts, Polyatomic Ions, Law of Conservation of Mass, Naming, Balancing Chemical Equations, Types of Chemical Reactions 6b. Stoichiometry Mole calculations, Gram-Formula Mass, Percent Composition 7. Gases Ideal vs. Real gases, Gas Laws 8. Thermochemistry Temperature, Heating and Cooling Curves, Specific Heat Capacity, Calorimetry, Heat of Fusion/Heat of Vaporization, Vapor Pressure 9. Solutions Classification of Matter, Solutions, Molarity and Concentration, Colligative Properties, Factors Affecting Solubility, Solubility Table (and rules) 10. Acids & Bases Properties, Arrhenius Definition, Nomenclature, pH scale, Indicators, General Reactions (especially neutralization), Titrations 11. Kinetics & Equilibrium Factors that affect the rate of reaction, Potential Energy Diagrams, Activation Energy, Endothermic vs. Exothermic Reactions, Enthalpy vs. Entropy Physical vs. Chemical Equilibrium, LeChatelier’s Principle 12. Redox Reduction vs. Oxidation, Assigning oxidation numbers, Half-Reactions, Voltaic vs. Electrolytic Cells 13. Organic Chemistry Carbon atom, Hydrocarbons (alkanes, alkenes, alkynes), Naming, Isomers, Uses, Functional Groups 14. Nuclear Chemistry Nucleus Stability, Nuclear Reactions and Nuclear particles, Fission vs. Fusion, vs. Artificial Transmutation, Half-Life, Uses and Dangers Natural TO BE SIGNED AND RETURNED BY FRIDAY 9/5 I have reviewed the Course Criteria sheet with my student and understand that the 1200 laboratory minutes must be completed to make my son/daughter eligible to take the New York State Regents Exam in June. Printed Name ____________________________________ Student Signature: ____________________________________ P/G #1 P/G #2 Parent/Guardian Signature: ____________________________________________________ Relationship ____________________________________________________ P/G Printed Name:___________________________________________________________ Parent/Guardian Daytime Phone: _______________________________________________ Evening Phone: _______________________________________________ Email: _______________________________________________________ Cell phone:____________________________________________________ What is the best way to reach you? ________________________When? ________________ Comments/Concerns: