Micro Exam 2 Study Guide
advertisement

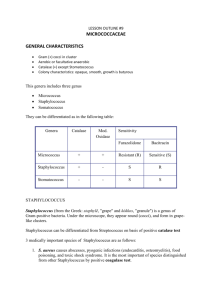
MDL 251 CLINICAL MICROBIOLOGY The Gram-Positive Cocci – Part I Staphylococcus species We encounter at least 3 clinically important species of staphylococci in the medical laboratory (refer to chapter 14 for background and description of the various species of Staphylococci) Staphylococcus aureus – Usually considered a pathogen when encountered, except when found in respiratory specimens where it is usually regarded as normal flora (reservoir in the nasal vestibule) Staphylococcus epidermidis – Highly opportunistic, but generally not considered a pathogen unless found in obvious association with disease (reservoir: skin surface and mucous membranes), Coagulase negative Staph. (CNS) Staphylococcus saprophyticus – Also opportunistic, and considered pathogenic when found in conjunction with urinary tract infections (UTI)/ pyelonephritis in pregnant women (reservoir: skin, urogenital tract), Coagulase negative Staph. (CNS) Many other species exist that are not further identified in the clinical lab due to lack of clinical significance. Identification of Staphylococcus Blood Agar Creamy, white or light gold; buttery-looking May be beta hemolytic (species dependent) Chocolate Agar Similar to growth on blood agar (no hemolysis) Mannitol Salt Agar - Growth MacConkey Agar - NO GROWTH!! Selective agars: PEA, CNA, CHROMagar Staphylococcus species Identification schema: The catalase and coagulase tests are key for identification of the staphylococci. Study these on pages 239-241 Catalase- Production of bubbles when organism mixed with Hydrogen Peroxide (H2O2). See Page 201 for procedure Coagulase- presence of enzyme causes fibrin clot formation in presence of S. aureus. See Page 203 for procedure. Staphylococcus aureus Reservoir is in the anterior nares; colonizes damaged skin Presence usually suggests pathogenicity, except in respiratory cultures Resistant to heat, desiccation, high salt concentration and alcohol Recovery strategy Grows very well on blood agar (BA) and chocolate agar Also grows on polyethyl alcohol (PEA) agar Also CNA, PEA agar and mannitol salt agar (high salt concentration) Growth at 35°C in CO2 in 18- 24 hours. Specimens of choice: Blood, Urine, Wounds Acquired resistance to methicillin – MRSA Methicillin resistant Staph aureus (MRSA) increasing in frequency, due to highly prescribing methicillin and other penicillins in 1960’s. 2005- 60% of S. aureus isolates were MRSA, some resistant to up to 20 different antibiotics 1 May use CHROMagar Staph. aureus Identification strategy Recover large white colonies from BA (often beta hemolytic) Gram stain= gram positive cocci in clusters Catalase test – positive (streptococci are negative) Coagulase test – positive for S. aureus; negative for S epidermidis and other CNS If slide test for bound coagulase is negative, must then perform a tube test for free (unbound) coagulase Produces a variety of enzymes and bioactive proteins Presence of these may determine pathogenicity and virulence Tend to vary among different strains S. aureus - produces localized suppurative (pus-forming) infections facilitated by a variety of enzymes and bioactive substances (see examples below) Protein A Major antigenic component, located in cell wall of S. aureus Has anti-phagocytic and anti-complementary properties Lowers host resistance to other organisms Coagulase Produces a fibrin clot at site of infection, trapping additional organisms Neutralizes bactericidal effects of normal serum Alpha toxin and hemolysins Breaks down RBCs Inactivates leukocytes Increases tissue cell permeability May be dermonecrotic Polysaccharide capsule – slime layer, biofilm Toxins Leukocidins and hemolysins inactivate antiphagocytic cells Enterotoxins cause disruption in Na/K pump in bowel producing diarrhea and vomitting Exfoliative toxin causes skin depolymerization and peeling TSST-1- Toxic Shock Syndrome Toxin Aggressins – a class of enzymes which collectively act on host tissue to establish bacterial infections and relate to virulence Hyaluronidase – loosens tissue via depolymerization; related to septicemia Lipase – acts on oils and fats in skin; related to furuncles and carbuncles DNAse – degrades host DNA Staphylokinase – desolves fibrin clots; inactivates plasminogen (may be utilized as an anti-clotting medication) Biofilms Diseases produced by S. aureus (see p. 234-235) Toxic Shock Syndrome (TSS) TSST-1- Toxic Shock Syndrome Toxin- produced by organism Causes fever, vomiting, diarrhea, desquamation, hypotension May be life-threatening via progression to uncontrolled shock and sepsis, death Furruncles Boils; limited subcutaneous involvement Carbuncles- Focus of deep-seated abscess, often with multiple drainage sites 2 Associated with genetic predisposition and/or immune compromise (George Washington suffered endless misery from these) Common in diabetic patients Scalded Skin Syndrome (Ritter’s Disease) Mostly in children/ neonates and facilitated by exfoliative toxins May manifest as blister, or desquamation of skin, which may be painful; strain specific Lesions are sterile – cannot recover organism from these; classic intoxication MRSA INFECTION Bacteremia Presence in blood but no reproduction in blood May cause endocarditis; fatality is about 50% Petechiae and positive blood cultures Septicemia Bacterial reproduction in blood Usually involves neonates or the debilitated elderly Osteomyelitis From trauma, fractures, bruises; may involve marrow Most common in children <12 years of age Usually found at ends of long bones or in spinal column The Staphylococci (cont.) Pyelonephritis From kidney stones, previous bacteremias Impetigo Bullous (blister), exfoliative bacterial dermatitis Usually nosocomial in origin Produces septic lesions; do not confuse with scalded skin syndrome Food Poisoning From enterotoxins; produces sudden onset of nausea, diarrhea and vomiting; NO fever Types A and B enterotoxins Common 2-6 hours after picnics (eggs, seafood, pastries) Other Staphylococcal infections S. epidermidis, S.haemolyticus and S.lugdunensis infections: Associated with medically implanted devices, nosocomial spread of resistant isolates, opportunistic S. saprophyticus infection UTI-females Micrococcus species Gram positive cocci in clusters Colonies: small, opaque, pigmented (yellow), nonhemolytic Catalase=positive Coagulase=negative Microdase=positive (see p. 215) Usually a contaminant. Rarely implicated in infections Treatment Resistance to every therapeutically useful antimicrobial has been described. Detailed study of specific antimicrobials and their mechanisms will be explored in Micro 2. 3 Key Terms Catalase - Bacterial enzyme that breaks down peroxides with liberation of free oxygen Coagulase – protein enzyme that enable conversion of fibrinogen to fibrin, used to differentiate S. aureus Microdase – Modified oxidase test used to differentiate Micrococcus spp. from catalase positive staph spp. Novobiocin – differentiates S. saprophyticus MRSA – Methicillin-resistant S. aureus TSST – Toxic shock syndrome toxin, S. aureus Hyaluronidase - It is often speculated that Streptococcus and Staphylococcus pathogens use hyaluronidase as a virulence factor to destroy the polysaccharide that holds animal cells together, making it easier for the pathogen to spread through the tissues of the host organism. Exotoxins – toxin secreted by bacteria Enterotoxins – protein exotoxin released by a microorganism that targets the intestines Aggressins – Any substance produced in the body by a pathogenic bacterium that enhances the virulence of the bacteria Scalded Skin syndrome - The syndrome is caused by S. aureus and causes detachment within the epidermal layer. Impetigo – highly contagious staph infection (aureus or less commonly pyogenes), causing lesions and scabbing often seen on arms, legs, and face. Furuncles – another S. aureus infection resulting in boils at a hair follicle The Gram-Positive Cocci – Part II Streptococcus and Enterococcus species Streptococcus species Streptococci are ubiquitous in clinical specimens, and are notorious opportunistic pathogens. Scan chapter 15, but do not become intimidated with the myriad of details presented in this chapter. We will begin by focusing on Four clinically relevant streptococci and their close cousins the enterococci: Streptococcus pyogenes (Group A strep; Beta-hemolytic strep) Streptococcus agalactiae (Group B strep; Beta-hemolytic strep) Streptococus pneumoniae (Alpha-hemolytic strep; the pneumococcus) Streptococcus - Group D (Alpha or non-hemolytic strep) Enterococcus faecalis (Alpha,non-hemolytic or beta) Description Partial lysis of red cells around colony; greenish discoloration around colony Complete lysis of red cells around colony; clear area around colony No lysis of red cells around colony; no change in agar Small area of intact red cells around colony surrounded by a wider zone of complete hemolysis Identification schema: The catalase test separates streptococci (catalase negative) from the staphylococci (catalase positive) Hemolysis: The more pathogenic species often display beta-hemolysis (see page 87, Photos p. 94) Other biochemical tests include: growth in 6.5% NaCl (p. 226) and growth in bile-esculin media (p. 199 ) 4 Most strains of streptococci are fastidious and have particular nutritional requirements. Identical strains may cause more than one disease, while a single disease may be caused by immunologically different strains. Also, one antigenic type may show association with more than one single infection Streptococci may be classified on the basis of Hemolysis (alpha= green, beta= clear, gamma= none) Biochemical reactions Ability to tolerate NaCl and/or bile salts Sensitivity to Optochin & Vancomycin Lancefield groupings Immunologically (ASO, Anti-DNase, Anti-streptokinase, anti-hyaluronidase) Source of recovery Streptococcus species (cont.) Most can be grouped according to Lancefield’s scheme (except pneumococci) and certain other non-pathogenic strains); Grouped according to carbohydrates (C-substances) in cell wall. Of the Beta Hemolytic strains Group A – S. pyogenes (anginosus group Group B – S. agalactiae Group C – opportunistic pathogens found in humans and horses Group D – alimentary canal of man and animals (usually non hemolytic) Note: Enterococci – originally members of the Group D strep; now a separate genus- may be alpha, beta or non-hemolytic Many species of alpha or non-hemolytic streptococci inhabit the normal skin, alimentary canal, mucous membranes and oral region; presence does not necessarily imply infection Most beta-streptococci do not usually occupy healthy tissue; presence almost always suggests pathogenicity Streptococcus pyogenes (Group A streptococci) Identification Beta-hemolytic and causes acute human infections; most beta-hemolytic clinical isolates will be S. pyogenes; one of most aggressive pathogens encountered. PYR test – positive ( pg 225) Often identified serologically (Lancefield precipitin test originally, now latex agglutination or coagulation) rather than biochemically (see page 136 and 258) Presence always suggests pathogenicity Relatively unresponsive to most biochemicals; sensitive to vancomycin Specimens of choice: Wounds, Respiratory, Urine Streptococcus pyogenes (cont.) Many strains produce a variety of virulence factors (see p. 250) M-protein – a major virulence factor Shared by all strains of S. pyogenes May be lost if repeatedly subcultured in lab Has anti-phagocytic properties via hyaluronidase in their capsules Facilitates attachment to tissues Hemolysins – Streptolysin S: Not produced by all strains; produces hemolysis of RBCs and is leukocidal; non-antigenic Streptolysin O: Damaged by oxygen and is reversibly deactivated; is cardiotoxic and leukocidal; is antigenic; basis for ASO test used to detect rheumatic fever Streptokinase 5 Fibrinolysin; destruction of fibrin clots Plasmin deactivator May be administered medically- used as “clot buster” for strokes/ MIs Erythrogenic toxin – Streptococcal pyrogenic exotaxin (SPE) Scarlet fever toxin, result of Streptococcal pharyngitis: causes rash of face & upper trunk. Toxin is immunogenic and immunity ensues against the toxin, not the organism. Also Toxic Shock Syndrome- renal & respiratory failure, rash & diarrhea. S. pyogenes (cont.) – Disorders produced by Group A streptococci include watery, spreading lesions (suppurative) which are highly communicable and infectious Scarlet fever – Pharyngeal involvement with rash on face & upper trunk Necrotizing fasciitis and myositis – “flesh-eating bacteria” Acute Pharyngitis – Classic sore throat in children; may spread to other organ systems if not treated Most classic bacteria causing sore throats, skin infections. Also “flesh-eating bacteria”- deeper tissue & organ involvement Perinasal sinusitis – From unresolved pharyngitis Also leads to otitis media, mastoiditis (ear canals), cervical adenitis (inflamed lymph nodes) Erysipelas – localized inflammation of skin & hot, red lesions on face & head Diffuse pyoderma produced by streptococcal toxin Impetigo – Diffuse maculopapular rash; staphylococci may be a predisposing factor Rheumatic fever – post-streptococcal disease Follows unresolved pharyngitis and/or scarlet fever Detected by ASO test Glomerulonephritis – post stretococcal disease Follows unresolved pharyngitis Deposition of Ag-Ab complexes in kidney resulting in loss of renal function Streptococcus agalactiae Often referred to as Group B streptococci, and are the second of two frequently encountered beta-hemolytic streptococci (the first was S. pyogenes); see pp 248-251 for a discussion of Group B streptococci S. agalactiae is a frequent cause of neonatal sepsis and may be isolated from the normal vagina; its presence is always assumed to denote pathogenesis, but otherwise may be part of the normal vaginal flora in the non-pregnant female S. agalactiae (along with E. coli and Listeria monocytogenes) is the most frequent cause of fatal sepsis and meningitis in the newborn infant Prospective mothers are routinely tested for S. agalactiae as a precaution; Unfortunately pregnant women may carry the organism, but be culture-negative May also cause rare cases of UTI, pneumonia, endocarditis, arthritis, osteomyelitis and other skin & soft tissue infections in immunocompromised patients Capsular material interferes with phagocytic activity and complement cascade activation Specimen of choice – vaginal; blood; CSF Streptococcus agalactiae (cont.) Beta hemolytic, Morphologically similar to Group A streptococci. Usually a smaller beta zone. PYR test = negative 6 Demonstrates at lease three different types of carbohydrate envelope antigens – I, II and III, and may be identified on the basis of serological tests (latex agglutination testing) Also may be differentiated from Group A streptococci on the basis of the CAMP test (Christie, Atkins, Munch-Peterson), which is an old test, but still performed in certain smaller laboratories (see p. 200): Latex Agglutination testing for Lancefield grouping of beta hemolytic Streptococcus Other Beta Hemolytic Streptococci Groups C, F and G Identified serologically (Lancefield precipitin test originally, now latex agglutination or coagulation) rather than biochemically Recovered from wounds, respiratory ,blood. Streptococcus pneumoniae Streptococcus pneumoniae is often referred to as one of the viridans streptococci due to its production of alpha hemolysis, which denotes an incomplete clearing of red blood cells when cultured on blood agar; also referred to as the pneumococcus A causative agent of subacute bacterial endocarditis (SBE) and/or septicemia and frequently gains entrance to the bloodstream from virtually any portal, especially the nasopharyngeal region where it may be regarded as normal flora. Common following rheumatic fever (valves damaged), or in abnormal heart. Specimen of choice is blood Causative agent of bacterial (lobar) pneumonia, occurring as a sequellum to previous viral infection – especially in alcoholics, smokers, and debilitated Specimen of choice is deep cough and/or transtracheal aspirate Pneumococcal meningitis – may initially involve middle ear (otitis media); also from pharyngitis, sinusitis CSF is specimen of choice; sensitive to cold, therefore keep specimen warm Specimens may be collected from other opportunistic sites/infections Gram stain appears as gram positive cocci in pairs (lancet shaped) Colonies tend to dip down in the center and resemble a doughnut (umbilicated) as they age Colonies may be mucoid if polysaccharide capsule present (Quellung test- determine serologic testing) Auto-hemolysis produces characteristic colonial appearance on blood agar, resembling volcano - concave centers ID based on optochin( pg. 222) and bile solubility (pg. 199) tests Sensitive to vancomycin Vaccine available to prevent infection from S. pneumoniae (recommended for elderly, aspleenic, sickle cell, diabetes, HIV, immunocompromised) Enterococcus and Group D Streptococcus Composed of a large number of saprophytes and opportunistic pathogens; their presence in clinical specimens does not necessarily suggest pathology (except blood). May be either alpha, or nonhemolytic . Enterococcus spp. may be alpha, beta or gamma Enterococcus may be separated from Group D streptococci, and these from other Streptococcus spp. (viridans ) on the basis of bile-esculin hydrolysis, and growth in 6.5% NaCl broth. 7 Enterococcus testing Salt tolerance (pg 200) Bile Esculin (pg 226) Enterococcus and Grp D Strep Responsible for UTI (specimen – urine); or SBE (specimen – blood) Most strains considered normal flora in respiratory and alimentary canal Enterococci were once counted among the Group D streptococci; now comprises the separate genus Enterococcus (E. faecalis and E.faecium) Enterococcus spp. have acquired resistance to many commonly used antibiotics Enterococcus Vancomycin resistance has become more common --VRE Key Terms Hemolysis – visible breakdown of red blood cells on blood agar Pyr test – Pyrrolidonyl arylamindase test. S. lugdunensis, S. pyogenes, and Enterococci spp are positive. Lancefield groups – groups beta hemolytic bacteria based on carbohydrate composition of bacterial antigens found on cell walls. Camp test – used to identify beta hemolytic streptococci. Bile esculin – used to identify Enterococci and Group D Strep. Salt Tolerance – used to identify Enterococci Optochin – used to identify S. pneumoniae Bile Solubility – used to identify/confirm S. pneumonia ASO - Anti-streptolysin O (ASO or ASLO) is the antibody made against streptolysin O, an immunogenic, oxygen-labile hemolytic toxin produced by most strains of group A and many strains of groups C and G streptococci. The main function of streptolysin O is to cause betahemolysis. Streptokinase – enzyme secreted by several species of streptococci that activate human plasminogen SPE – exotoxins produced by S. pyogenes Scarlet Fever – infection of S. pyogenes from erythrogenic toxin Rheumatic fever – inflammatory disease post S. pyogenes infection. Neonatal sepsis – systemic bacterial infection of newborn after exposure to flora of birth canal VRE – Vancomycin-resistant Enterococci Gram-Positive Rods: Bacillus, Listeria, Corynebacterium and Related Organisms The gram-positive rods are comprised of many genera, some of which are aerobic spore-forming, and non spore-forming; many of which produce serious disease . Several genera will be covered here, others in subsequent lectures. Bacillus species: Aerobic spore-forming rods (see Ch. 16) Listeria monocytogenes: Aerobic non spore-formers (see Ch. 17) Corynebacterium species and related organisms: Aerobic non spore-formers (see Ch. 17) Eripselothrix, Lactobacillus and Gardnerella : Aerobic (Ch. 18) You are only responsible for the chapter sections that are related to the above organisms Bacillus species At least three B. species are encountered clinically: B. cereus, B. subtilis, and B. anthracis. Produce spores which are metabolically inactive and resistant to heat & chemicals. Are aerobic or facultatively anaerobic, grow on sheep BA, and are large gram positive or gram variable rods, with spores appearing as holes inside rods. Specific causative agent of anthrax 8 B. cereus: Recovered from soil and grains; Causative agent of food poisoning (intoxication) producing a self-limiting, non-fatal nausea and vomiting, similar to that caused by S. aureus Usually associated with partially cooked fried or boiled rice May cause septicemia in drug abusers and/or those on hemodialysis B. subtilis: Recovered from soil: Rare opportunistic causative agent of wound and eye infections; frequently overlooked and/or under-reported Bacillus anthracis Large boxy gram-positive spore-forming rods with subterminal endospores; forms large, nonhemoltyic medusa-head colonies on BAP. Sporulates under aerobic conditions. Production of capsules which impart anti-phagocytic properties Spores formed only in air; highly resistant; ingestion/inhalation and germination Forms “strings of pearls” (chains of spherical bacilli) when incubated for several hrs on agar containing low concentration of penicillin Lab personnel must work under Biosafety Level 3 conditions Pathology Pathogenicity due to overwhelming septicemia, capillary damage and death Produces toxin composed of multiple components Multiple clinical manifestations occur in man Cutaneous anthrax – formation of papule to slack scar (Black Eschar) on skin at site of spore penetration; usually from contaminated hides, wool or hair; 20% mortality if untreated (from toxemia) Characterized by generalized hemorrhage and edema Rarely progresses to meningitis and/or pneumonia Pulmonary anthrax (Wool Sorter’s Disease): Severe respiratory infection with nearly 100% mortality whether treated or not; begins with malaise & mild fever & nonproductive cough, progressing to respiratory distress, massive chest edema, cyanosis & death Very rapid dissemination, initially producing bronchopneumonia Severe mediastinitis (area separating the lungs); involving many lymph nodes Hemothorax, septicemia and/or meningitis Rapidly fatal multiple organ failure Gastrointestinal anthrax: Rare form of Wool Sorter’s Disease produced by eating raw meat and/or massive numbers of spores Ingestion of spores; affects either oropharyngeal or abdominal area Most patients die from toxemia & overwhelming sepsis. Bioterrorism worry- Post Office scare of 2001-2002. Most likely bioterroristic organism to use for agents of mass destruction. The Corynebacteria Loose knit group of irregularly staining gram-positive non-spore-forming pleomorphic rods, many with fastidious growth requirements; most do not cause disease and are opportunistic pathogens. “Coryne” is Greek for club- club-shaped rods. Gram positive short or slightly curved rods with rounded ends- some with rudimentary branching- some in parallel rows (palisades) or resembling Chinese letters (V, L & Y forms) Main pathogen: C. diphtheriae Other Corynebacteria may produce a variety of diseases, generally associated with bacteremias, septicemias, endocarditis and/or meningitis The term “diphtheroids” is used to describe non pathogenic corynebacterium (not diphtheriae) 9 Corynebacterium diphtheriae (Klebs- Loeffler’s bacillus) Causative agent of diphtheria – leathery throat lesion (pseudomembranous “diphth”) Man is sole habitat; mucous membrane of nose and throat; skin & vagina. Some are asymptomatic carriers. Pathology Represents a true intoxication; toxin only is disseminated, acting on PNS and heart Toxigenic strains infected by specific bacteriophage that carries toxic gene Incubation 1-7 days; low fever, pallor, tachycardia, weakness Produces a fibrinous exudate and necrotic tissue; may erode epiglottis Can extend to larynx and close off trachea Peripheral vascular collapse and death Recovery does not impart immunity Sequellae include myocarditis and chronic peripheral neuritis Also Cutaneous form of diphtheria- nonhealing ulcers with membrane formation Corynebacterium diphtheriae Culture for C. diphtheriae is on: Tinsdale’s agar (black colonies with dark brown halo) Cystine-tellurite BA (black or gray colonies) Also Loeffler’s medium- contains serum & egg- shows metachromatic granules in cells Testing for diphtheria: Biochemical testing, whole-cell fatty acid analyses, cell wall diamino acid analysis or 16S rRNA gene sequencing- reference labs only Toxin testing for diphtheria using guinea pig lethality testing, immunodiffusion testing using Elek plate, tissue culture cell test, and PCR test Treatment: antitoxin to neutralize diphtheria toxin plus penicillin or erythromycin to kill organism About one person in 10 who gets diphtheria dies of it. Diphtheria is more severe for those under 5 and over 40 years of age. Most cases have occurred in nonimmunized (or inadequately immunized) individuals. Vaccine: multidose diphtheria toxoid (inactivated)- begun in infancy, boosters every 10 years (Diphtheria & Tetanus) Exposed persons: treat with single dose IM penicillin or 7-10 day course oral; follow-up throat cultures at 2 weeks (repeat 10 day if positive); also be revaccinated. Listeria monocytogenes Causes profound neonatal sepsis and death; primarily disease of persons under 1 month, and over 40 yrs of age; organism easily recovered from soil, widely distributed in nature, occasionally found in human GI tract. Dangerous due the ability to survive inside phagocytes due to formation of Listeriolysin toxin; short gram positive rod occurring singly or in short chains resembling streptococci Infection by ingestion of contaminated food, such as meat and dairy products. Also colonized mothers pass organism onto fetus. Entry usually from GI tract to blood, and sometimes, to meninges. A peculiar property that affects its food-borne transmission is the ability to multiply at low temperatures. The bacteria may therefore grow and accumulate in contaminated food stored in the refrigerator. So it is not surprising that listeriosis is usually associated with ingestion of milk, meat or vegetable products that have been held at refrigeration temperatures for a long period of time. 10 Initial symptoms may include flu-like symptoms, backache, vomiting diarrhea, headache. Meningitis accounts for 75% of cases of Listerial infections; other clinical manifestations exist . Often complicated by encephalitis, a pathology that is unusual for bacterial infections . When listeria meningitis occurs, the overall mortality may be as high as 70%; from septicaemia 50%, from perinatal/neonatal infections greater than 80% in the fetus. CNS listeriosis in pregnant mothers and otherwise healthy individuals may be asymptomatic Septic military granulomatosis causes cardiorespiratory distress in military recruits Vomiting, diarrhea, dark red skin papules, hepatosplenomegaly Abscesses of liver, spleen, adrenals, lungs, pharynx, GI, CNS Oculoglandular listeriosis Severe conjunctivitis, corneal ulceration May occur at any age Most susceptible: elderly, immunocompromised or if underlying disease is present; (transplant patients, diabetics, Hodgkin’s disease) ISOLATION Clinical specimens: CSF, blood, placenta, fetal tissue, stool Good growth on 5% BAP (beta hemolytic) Oxford media ( selective) black colonies – for non-sterile sources IDENTIFICATION Small, Gram-positive rods, which are sometimes arranged in short chains. In direct smears they may be coccoid, so they can be mistaken for streptococci. Longer cells may resemble corynebacteria. Presumptive ID by observing motility by direct mount (end-over-end tumbling) in nutrient broth at RT for 1-2 hrs. Alternate is umbrella-shaped pattern from overnight RT incubation in tube of semisolid media. Catalase (+) , esculin( +) Immunocompromised & pregnant women should avoid eating soft cheeses such as Mexican-style, brie, feta, Camembert, & blue-veined cheese, and reheat hotdogs & cold cuts before eating PCR assay available for testing food proucts Ampicillin & penicillin are treatment options Listeria species Erysipelothrix rhusiopathiae Carried in the intestinal tract of turkeys and swine ( also rabbits, sheep, birds) Causes erysipeloid, localized cellulitis, following a bite in an individual handling animals or animal products. Butchers and veterinarians May become disseminated and lead to endocarditis Identification -short gram pos rod -growth on BAP -catalase (-), H2S (+) -commercial systems (API) Penicillin is the treatment of choice Lactobacillus Normal flora of human mouth, GI tract and female genital tract Usually a contaminant. May cause infections in the immunocompromised. Gram stain: gram pos rods in chains (fig 18-1) (called an aerotolerant anaerobe) 11 Grows well on BAP(alpha), Choc, CNA Cat.= neg Mac=NG Gardnerella Normal vaginal flora Causes Bacterial vaginosis, UTI Growth on BAP,Choc,CNA, HBT (beta) (fig 18-2) Gram stain: pleomorphic Gram variable or gram neg rods and coccobacilli “clue cells” on saline wet mount or gram stain Clue cells- stipled epithelial cells, indicating infection 1. Which simple test will separate Staphylococci from Streptococci? Catalase positive organisms are Staphylococci sp., while negative would be Streptococci sp. 2. Which tests will separate S.aureus from all "coagulase negative staphylococci" like S. epidermidis? Coagulase. S. aureus is positive. 3. How can you differentiate Micrococcus from coagulase negative staphylococci like S. epidermidis? Micrococci are Microdase positive while Staphylococci are negative. 4. Staphylococci are considered resistant to Novobiocin if the zone is less than 16mm. 5. Which organism may give a pseudocatalase positive result? Enterococci 6. Why is it useful to perform both a slide and tube coagulase? Staphylococcus aureus contains both bound and free coagulase. The slide test confirms bound coagulase and the tube test is for free coagulase. Negative slide tests should be confirmed with the tube test. 12 13 14 15