The reactionary centers of carboxylic acids and their derivatives
advertisement

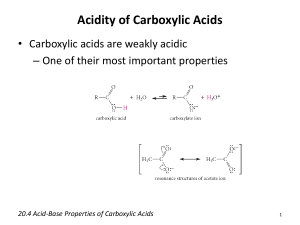
THE MINISTRY OF HEALTH OF THE REPUBLIC OF UZBEKISTAN TASHKENT MEDICAL ACADEMY "Approved" Vice-Rector of Tashkent Medical Academy prof. Teshaev O.R. "___" ____________2013 Year Department: BIOORGANIC AND BIOLOGICAL CHEMISTRY Subject: BIOORGANIC CHEMISTRY THE TECHNOLOGY OF TRAINING ON THE LABORATORY LESSON TOPIC: THE REACTIONS OF NUCLEOPHILIC SUBSTITUTION SN OF CARBOXYLIC ACIDS AND THEIR FUNCTIONAL DERIVATIVES Methodical recommendations Tashkent - 2013 Author: Turayeva G.I. – assistant, department of bioorganic and biological chemistry TMA Reviewers: Alimkhodjaeva N.A. - associate professor, department bioanorganic, bioorganic and biological chemistry TashPMI Zaytseva O.A. – D.F. associate professor, department pharmacologies ТMА of of Considered and approved at a meeting of the DNC medical-biological disciplines __________________ 2013 minute № 2 Topic: THE REACTIONS OF NUCLEOPHILIC SUBSTITUTION SN OF CARBOXYLIC ACIDS AND THEIR FUNCTIONAL DERIVATIVES 1. Model of technology education Duration of studies: Number of students: 12-15 persons 135 minutes Department of bioorganic and biological Location of activities: chemistry 1. Introduction The structure of the training session 2. The theoretical part 3. Independent work of the student 4. Experimental part 5. The analytical part 6. Conclusion The aim of the study lessons: to acquaint students with individual representatives, chemical properties and biological value of carboxylic acids and their functional derivatives. To form practical skills of conducting a qualitative reaction on acetic acid, high-molecular fatty acid and oxalic acid. The student should know: Theory of chemical structure of organic compounds, types of reactions, mechanisms of the reactions, the structure features of a carboxyl group, characterizing properties and the biological significance of these compounds. The student should be able to: carry out qualitative reaction on acetic acid, receive high-molecular fatty acids in the form of calcium salts and open oxalic acid in the form of calcium salts. The pedagogical objectives: The results of educational activity: • To acquaint with the structure and Students: properties of carboxyl group • Aware of the structure and correctly write • Examine the reactivity and the formula of carboxylic acids and their reactionary centers carboxyl group functional derivatives • Study properties and structure of • Know the structure of the carboxyl group carboxylic acids and their derivatives • Know how to determine the reactivity and • Examine the SN reaction of reaction centers of carboxylic acids and carboxylic acids and their derivatives their functional derivatives • Examine the the reaction of the • Know how to carry out the reaction of the esterification and to acquaint with the esterification received product • Correctly write the equations of the • To study the reaction of hydrolysis of reactions of hydrolysis of esters and esters and tioesters and explain their tioesters biological value • Know S reaction of amides and nitriles N • Examine the SN reaction of amides carboxylic acids and nitriles carboxylic acids • Know the reaction of carboxylic acids Show the reaction of carboxylic acids with hydrazides 3 with hydraes To acquaint with anhydrides of carboxylic acids Show condensation reactions, carried out with the help of acetylecoferment A and their SN mechanism Show the reaction of receipt of halogen anhydrides acids Explain the biological significance of carboxylic acids and their functional derivatives The technique and methods of training: Forms of training: Means of training: Monitoring and evaluation • Know the reaction of anhydrides of carboxylic acids obtaining • Know the reaction of condensation, carried out with the help of acetylecoferment A and their SN mechanism Know the value of these reactions in medicine Conversation, discussion, “Brainstorming”, drafting of graphic organizers, the decision of situational tasks and tests Individual work, work together in small groups Training manuals, training materials, presentation slides, handouts, methodical development step-by-step implementation experiments, Internet sites in the field of chemistry. Oral questioning, blitz-poll, situational tasks, tests 2. Technological map of learning activities based on interactive teaching method «Brainstorming» Stages of the Content of the activity work and time Teacher Student (135 minutes) 1-stage 1.1. Checks attendance Listen, write Introduction 1.2. Announces topic, purpose and expected (5 minutes) results. Listen to 1.3. Acquaints with the plan and the peculiarities of the training session. To clarify, ask 1.4. Introduces the criteria of assessment of questions knowledge and skills of students 2-stage 22.1. Checks the level of preparedness of Answer questions The students, ask questions, get answers on the topic. theoretical 2.2. Gives an explanation on unclear issues Listen, ask part questions (50 minutes) 2.3. Shows the video on the topic of chemical Examine and properties of carboxylic acids make conclusions and expand their understanding of 4 3-stage Independent work (20 minutes) 4-stage. Practical part (30 minutes) 5-stage Analytic part (20 minutes) 6-stage. The final part (10 minutes) properties of carboxylic acids 2.4. Sets out the scenario of interactive To clarify, ask teaching «Brainstorming» (5.3) and divides the questions, and are students into small groups (Appendix № 2). divided into small groups 2.5. Actuate activity of students on the basis of technology of training «Brainstorming»: Work in a group, assesses the work of the groups (individual ask questions, and participants), makes conclusions on mutual make a presentation, estimation participants. evaluate their own and their mutual 2.6. Sums up the results of the theoretical activities. part of the class. Listen to voice their opinion, 3.1. Verifies the implementation of Discuss, Express extracurricular (drawing up of textbooks, work their opinions, fulfill with literature and etc.) and class (filling in the the tasks of the IWS tables, graphic organizers, etc.) part of the self study work 4.1. Gives students the instructions on the Listening, asking implementation of the practical part of the class questions 4.2. Gives the assignment of students to Carry out the perform experiments laboratory work, the 4.3. Check the results of practical work results of the issue in the notebook, make conclusions, make a presentation of the 5 5.1. Sets for the solution of situational tasks Solve the and tests and evaluates tasks,tests, ask vague questions, discuss 6.1. Makes a conclusion on the subject, focusing students on the importance of the work on the follow-up and analyses the degree of achievement of the exercise. 6.2. Assesses the knowledge of students on the results of assimilation of the theory, practice and independent work and announces assessment. 6.3. Specifies the task to the next session, shall announce the list of literature and websites on the Internet Listen to Listen to Listen to Record 5 3. Motivation Carboxylic acids and their functional derivatives are included in the composition of a large number of metabolites and medicinal substance. For the study of the participation of these compounds in the process of vital activity of an organism need to know their structure and properties. 4. Interdisciplinary and intersubject connection Mastering of the topic students is based on the initial level of knowledge on organic and inorganic chemistry, biology, physics, mathematics, etc. The knowledge received during this session will be needed when studying the processes of metabolism in the course biochemistry, histology, normal and pathological physiology, pharmacology, clinical pharmacology, and also in the development of practical skills for working with the laboratory, and medicines. 5. The theoretical part 5.1. Control questions 1. Specify the reactionary centers connections to the carboxyl group, and then name the factors influencing their reactivity. 2. Write a general mechanism of the reactions of nucleophilic substitution of carboxylic acids and their functional derivatives. 3. Write the reaction of receipt of halogen anhydrides, amides, tioesters, esters and anhydrides of carboxylic acids, explain the mechanism of reactions. 4. Write the reaction of hydrolysis of derivatives of carboxylic acids. 5. Explain the reaction of condensation of carboxylic acids. 6. Tell us about the biological significance of carboxylic acids and their functional derivatives. 5.2. The workshop sessions Reactivity of carboxylic acids Compounds in which one or more hydrogen atoms are replaced by carboxyl group – СООН called carboxylic acids. Depending on the nature of organic radical RСООН carboxylic acids can be aliphatic, aromatic and heterocyclic. Carboxylic acids on the number of carboxyl groups are divided into one main, two main and polycarboxylic. For example, acetic СН3СООН and benzoic С6Н5СООН one main acid, malon СООН - CH2 - СООН and terephthalic СООН- С6Н4 - СООН two main acid. There are also saturated or unsaturated carboxylic acids. Carboxylic acids are relatively high acidity. Due to the-M-effect increases the mobility of hydrogen hydroxyl group. Due to the separation of a proton from the hydroxyl group is formed high-stability carboxylate - ion, communications and the charges in which aligned: 6 The reactionary centers of carboxylic acids and their derivatives The reactions of nucleophilic substitution are characteristic of carboxylic acids and their functional derivatives. In addition to electrofille the main and the weak CHacid centres available in aldehydes and ketons in a molecule of carbon acids and their functional derivatives is also associated with carbon atom of carbonyl group deputy X (potential leaving the group, nucleofuge), the ability to go in the form of anion X AC or conjugate acid HX . At the expense +I-effect of negatively charged oxygen atom at carbon atom in the product accession there is a partial negative chargeд which facilitates the separation of the outgoing group X. On the mechanism of the reaction proceeds in the presence of strong enough nucleophile Y and the good of the outgoing group X. Easy nucleophilic attack depends on the value of effective positive charge of the carbonyl group. In functional derivatives of carboxylic acids R-CO-X it increases with the-I-effect of a deputy X and decreases with increasing its +M-effect. Electrofile carbonyl carbon atom is reduced by +M-effect of the functional deputy X associated with the carbon atom of carbonyl group. For this reason, the nucleophilic reactions of derivatives of carboxylic acids are often essential acid catalysis - protronation of the oxygen atom of carbonyl group leads to the appearance of the full of positive charge at carbon atomа which facilitates the attack nucleofille. 7 Factors influencing on reactivity of carboxylic acids. The reactions of nucleophilic substitution of SN carboxylic acids and their functional derivatives. Getting halogen anhydrides Halides are the most active acilic reagents among derivatives of carboxylic acids. So, atsetilecholoride easily hydrolyses in water (atsetilate water) with heat release and the formation of acetic acid. Halides RCOHal receive action (PCl5, PCl3) or tionil chloride (SOCl2) on acid. Getting anhydrides. When replacing the hydrogen hydroxyl group of carboxylic acids on the balance of carboxylic acids are formed anhydrides acids. In practice anhydrides are as follows: 8 Acetic acid Anhydrides easily hydrolysable by with the formation of acids: There are still mixed anhydrides consisting of residues of organic and mineral acids. These include atsetilenitrate СН3СООNO2 and atsetilefosfate. Atsetilefosfate, substituted atsetilfosfate and substituted atsilfosfate play an important role in biochemical processes of the organism: Atsetilfosfate Substituted atsetilfosfate Substituted atsilfosfate (R - balance adenosine nucleoside, R’ radical carboxylic acid). Substituted atsilfosfates are metabolites, with participation of which in the body is the transfer of acil residuumes for hydroxyl, tionyl and аmino groups of various compounds. Esters and tioesters. Interaction of carboxylic acids from alcohols, leading to the formation of esters, i.e. the reaction of the esterification is the most important process of transformation of carboxylic acids and their functional derivatives. The esterification reaction in the absence of catalysts is very slow due to the already the low capacity of carbonyl groups in carboxylic acids to be subjected to nucleophilic attack due to the +M- effect of OH group reduction and positive charge at carbon atom. However, in the presence of mineral acids (sulfuric, HCl) reaction considerably speeded up. 9 The process of esterification is reversible. Below is the mechanism of this reaction. Complex esters RCOOR' and complex tioesters RCOSR' - the most widespread in the nature as derivatives of carboxylic acids. Many medicines contain in their composition of the ester group. Hydrolysis. Esters may be subjected to hydrolysis (in contrast to the reaction of the esterification) in both acidic and alkaline environments. Hydrolysis in the alkaline environment is irreversible and requires ekvimoleculare number of alkali. The following scheme of the mechanism is fair to alkaline hydrolysis not only esters, but also tioesters, halogen anhydrides, anhydrides and carboxylic acid amides. The cause of the irreversibility of hydrolysis in the alkaline environment is the formation of a stable masomer atsilate-ion. The most common representative of these substances in the body is acetyl coenzyme A (coenzyme of acetylation) – tioester of acetic acid and coenzyme A. Atsetilecoenzyme A in vivo serves as a carrier of acetyl groups on nucleofile substrates. 10 For example, with the participation of acetile coenzyme A precedes transformation of choline in acetilcholine. Atsetilecofermente A receives the following way: Amides of carboxylic acids. At the substitution of hydroxyl group carboxylic acid on aminogroup NH2 form amides of carboxylic acids: Amides of carboxylic acids have a weakly acidic and weakly basic property. Including of the electron acceptor acilic balance in the molecule of ammonia, leads to the appearance of diluted acidic properties. Under the influence of +M-effect amides are weakly basic properties compared with amines. The basic properties of amides acids are evident in the reactions of formation of salts with stronger acids: Amide group СN found in many biologically important О Н Substances, such as peptides and proteins. Nitriles of carboxylic acids. Nitriles of carboxylic acids are obtained by dehydration of acid amides: As well as under the action of cyanide of alkaline metals on haloidalkils: RBr KCN KBr RC N Hydration nitriles pass in an alkaline environment. It can be imagined as nucleophilic addition of water to a polarized triple connection nitrile with the 11 formation of the interim iminol, which are further changes in amide. Nitriles represent more weak bases than amides. They are deprived of their basic properties.When restoring nitriles into the corresponding amines: R – C N + 2H2 R – CH2 – NH2 One of the most important representatives of nitriles is acetonitrile CH3-С N. Condensation reactions. Condensation reactions, which are based on the ability of a carbonyl compounds to join the carbonyl group of the same or another carbonyl compounds are typical not only for aldehydes and ketones, but also for the derivatives of carboxylic acids in particular esters and tioefireов. Such reactions are of great biological importance. With their help in the body leads to the formation of new relations of the carbon-carbon. Indispensable participants of reactions by type альдольного accession in vivo are tioesters of carboxylic acids - derivative coenzyme A. In tioesters, compared with the usual esters due to the effective positive charge on carbonil carbon atom nucleophilic substitution in the atom is easier. For the same reason α - hydrogen atoms in tioesters more mobile and more active in the reactions of condensation. Biological importance of carbon acids. Formic acid НСООН is contained in the nettles and in separations of the ants, it is irritating to the skinу can cause burns. In medicine 1.25% alcohol solution of formic acid is used in the treatment of rheumatism. Acetic acid is a product of acetic fermentation of sugars. In medicine is applied in the form of salts. Potassium acetate СН3СООК - moderate diuretic agent; lead acetate (СН3СОО)2РЬ • ЗН20 - astringent, is used for inflammatory diseases of the skin and mucous membranes. Acetic acid is used for food preservation. Succinic acid has an unpleasant smell, is contained in gone bust oil and perspiration. Isovaleric acid is in the roots of valerian; a member of validol, valerian tincture. Has a calming effect. Sodium benzoate C6H5COONa - expectorant and poorly Dezin-фицирующее means.A large medical-biological importance is the derivative of carbonic acid urethanes, ureid - and guanid derivatives. 12 Urethanes - esters of carbomide acid H2NCOOR - have psychic effect. Some of them are used in nervous and mental diseases, showing sedative action. In particular, meprotane (meprobamat) is used as a tranquilizer. 5.3. Description of the method «Brainstorming» - (brainstorming-a storm of the brain), the Brainstorming method of collective generation of ideas solution of practical or scientific problems. -separate ideas from their evaluation and The main idea of the method analysis. The idea becomes a guide to all the actions of the leading questioning and to help participants to give as much as possible of new ideas. -express (generate) as much as possible The rules of questioning of their own ideas of its decision and write on the boar. Divides the participants into 3-4 groups Teacher and offers each mini-group problems (Appendix № 2) -encouraging participants (smile, encourage phrases, interjections); gently, but insistently prevent attempts to criticize other people's statements; -records every statement in an unmodified form - to overcome the psychological barrier in communication and providing moral assistance to the students the students are asked unexpected and original quiz questions from a current or предидушей topics (Appendix № 1) -to discuss and evaluate each the idea; After questioning -give the best ideas; 6. TASKS FOR INDEPENDENT WORK OF STUDENTS 6. 1. Presentation of independent work of a student, who has received a task on this topic: Annex № 3 6.2 Auxiliary means of training: graphic organizers Graphic organizer: «Lotus Flower» - figure: the Means of solving problems. Embodies the image of a lotus flower. It is based on the nine large squares, each of which is formed of 9 squares. Develops and activates the system, creative, analytical thinking. The rules of drawing up of the “Lotus Flower”: 13 When solving this type of graphic organizer students write in the centre of the central square of the basic idea. The concept of its decision recorded in the other eight squares, located around the central. From a practical point of view of convenience of this method lies in the fact that all the ideas are concentrated around the main ideas, and are presented on a single sheet that allows you to merge all the reactions of this cycle of the chemical properties of the compounds in a single scheme. It creates convenience for consideration of the large number of reactions on one sheet of paper, not leafing through the book, that up to attention. Graphic organizer «Lotus Flower»: SN reaction of carboxylic acids HSCoA РCl3 PCl5 SOCl2 Н3РО4 P2O5 C2H5OH Example: Graphic organizer «Lotus Flower»: SN reaction of carboxylic acids 14 7. PRACTICAL TRAINING TASK Practical task will be executed in minigroups The technological scheme of the method Teacher Students I stage - Briefing Briefly presents a practical task, using not Listen to and record. difficult terms to explain the purpose of its carrying out. II stage -Getting acquainted with the instruction of the method Providing students with instruction on the Get acquainted with the instruction on implementation of practical tasks. the implementation of practical tasks III stage - Implementation of tasks In the beginning of the operation, make Prepare the necessary equipment and sure whether they have everything you reagents, and begin to implement it. 15 need, whether the issues relating to the process. To watch the progress of the job. IVstage – presentation of the results For a few minutes before the expiry time, Mini groups present the results of the warn of job completion. work and present V stage - The final review According to the obtained data, interpret the results and draw conclusions Experience № 1. The opening of the acetic acid Objective: to study the reactions of nucleophilic substitution of carboxylic acids Not fulfilled Completely (0 points) correctly Stages Actions 1. 2. 3. Take a clean dry tube Put in a test tube 10 drops of acetic acid Add 5-6 drops of water 0 0 0 0,5 0,5 0,5 4. With the help of litmus paper to determine the environment Add 10 drops of solution of sodium hydroxide To the mixture add 10 drops of 1% solution FeCI3 Heated on the stove Write the equation of reactions and conclusions in a notebook, explain, what is the practical application of this reaction has Total: 0 1,0 0 0,5 0 0,5 0 0 0,5 3,0 0 7,0 5. 6. 7. 8. Experience № 2. The formation of insoluble calcium salts of higher fatty acids. Objective: to study the reactions of nucleophilic substitution of carboxylic acids. Not fulfilled Completely (0 points) correctly Stages Actions 1. Take a clean dry tube 2. Put in a test tube 10 drops of a soap bubble solution 3. Add 2 drops of the solution CaCl2 4. Mix 5. Write the equation of the reaction and 0 0 1,0 1,0 0 0 0 1,0 1,0 2,0 16 conclusions in a notebook, explain, what is the practical application of this reaction has Total: 0 6,0 Experience № 3. Opening oxalic acid form of calcium salts. Definition of acetic acid Objective: to study the reactions of nucleophilic substitution of carboxylic acids Stages Actions 1. Take a clean dry tube 2. Put a few crystals of oxalic acid in the tube 3. Add 5-10 drops of water 4. Add to solution 2 drops of CaCl2 5. Write the equation of the reaction and conclusions in a notebook, explain, what is the practical application of this reaction has Total: Not fulfilled (0 points) Completely correctly 0 0 1,0 1,0 0 0 1,0 1,0 3,0 0 7,0 8. The analytical part 8.1. Situational tasks: 1. To obtain the functional derivatives of carboxylic acids do not take the carbon acid and its chlorangadrate. Write the reaction of the receipt of atsetamide of chlorangadrate carboxylic acid, explain the mechanism of reaction and confirm the reason why it is better to use chlorangadrate, and not the acid. (Answer: the Reaction goes on SN mechanism. Activity chlorangadrate is explained by the fact that chlorine is the good of the leaving group. Positive mezomer effect of chlorine less than the hydroxyl group, acidity more, and the positive charge at carbon above and SN reaction is easier.) 2. In the human body many reactions of carboxylic acids are held with the participation of tioesters. For example, the transformation of choline in acetylcholine, forming of citric acid from sorrelvinegar acid and atsetilecoenzyme A. Write the reaction of transformation of choline in acetylcholine, explain the mechanism of reaction and confirm why nature as a substrate or reagent chose tioesters, and not esters of carboxylic acids. (Answer: the Reaction goes on SN mechanism. Activity tioesters is explained by the fact that SR group is a well leaving the group. Positive mezomer effect SR group less than the hydroxyl group, the radius of the sulphur 17 greater than the radius of oxygen, so the acidity more, and the positive charge at carbon above and SN reaction is easier.) 3. In the body acilfosfate delivers acil group of alcohol, tiols and substances containing aminogroupe. Write the reaction of the acetiladenilate and coenzyme A. Explain the mechanism and why these substances exist in the form of phosphate anhydride. (Answer: the reaction goes on SN macchanism.So as a phosphate group is a well-outgoing group, anhydrate group easily hydrolyses and the gap anhydrate link leads to the formation of a large amount of energy and increases the energy potential of the process.) Acetiladenilate Coenzyme A Atsetilecoenzyme А АМF 8.2. The decision of the tests 1. Tests with one correct answer: 1. Depending on the nature of organic radical carboxylic acids can be: A) one main B) two main C) three main D) a lot of the basic E) aromatic* 2. During the interaction of carboxyl group of carboxylic acids with urea are formed: A) ureide acid* B) urea acid С) amides D) amino acids E) amines 3. Functional derivatives of carboxylic acids type of R-C(O)-NН2 are called: A) azides B) amines C) amides* 18 D) salt E) amine derived 4. That is the product of the reaction of acetic acid and ethyl alcohol? A) simple ester B) half atsetal In) ethyl acetate* D) acetic ethyl E) acetal 5. The reaction of carboxylic acids, going on C-H acid center: A) eterification B) atsetilization C) forming of amides D) condensation* E) anomerization 2.Tests with several correct answers: 1. Specify the 4 factors that increase the activity of carboxylic acids and their derivatives in the reactions of SN: 1. the presence of electron acceptor deputies in the hydrocarbon skeleton of molecules* 2. the small negative inductive effect of deputies 3. a large amount of positive mesomer effect of deputies 4. the neutrality of the environment 5. the large amount of negative inductive effect of the deputy of the derivatives of carboxylic acids* 6. decrease of the positive mesomer effect of deputy* 7. availability elektron donor deputies in the hydrocarbon skeleton 8. the presence of acid catalyst* 2. Specify the 5 compounds, participating in the reaction of the saponification: 1. isopropylamine 2. diethyl ester 3. acetilchloride* 4. benzonitrile* 5. aniline 6. ethyl acetate* 7. dimetilketone 8. formaldehyde 9. atsetilecoensim A* 10. propilamide* 3. Specify the 4 factors which depend on the ability of the derivatives of carboxylic acids to hydrolysis: 1. and the positive charge on the carbonil carbon* 2. the negative charge on the carbonil carbon 3. the nature of the substituents in carboxylic acid* 4. the value of positive inductive effect of the deputy 5. the amount of negative mesomer effect of the deputy 19 6. the amount of negative inductive effect of the deputy* 7. the magnitude of positive mesomer effectof the deputy* 8. strength of the carbon - hydrogen 4. Name the 5 most important functional derivatives of carboxylic acids on - COOH group 1. salt* 2. amines 3. esters* 4. amides and substituted amides* 5. hydrates 6. complex tioesters* 7. esters 8. disulfides 9. alcohols 10. anhydrides, halides* 5. Specify the 4 of reaction centre in carboxylic acids and their derivatives 1. electrofile center* 2. the potential outgoing group* 3. OH acid center 4. nucleophilic acid center* 5. electrofile the main center 6. NH-acid center 7. CH-acid center* 8. n-the main center 6. Select 4 responses, describing the stages of the reaction mechanism esterification 1. interaction carboxylic acids with proton-catalyst* 2. dissociation of molecules of water* 3. formation of carbanion 4. output catalyst with the formation of ester* 5. electrofile attack on carbanion 6. connection the molecules of water 7. the formation of hydrate 8. alcohol nucleofile attack * 7. Select 4 compounds, which are derivatives of the carbonic acid 1. urea* 2. ureid* 3. glycine 4. stearate 5. phosgene* 6. oliate 7. urethanes* 8. nucleic acids 8. Select 5 acids, which are одноосновными 1. oil* 2. lactate* 20 3. oxalic 4. acetic* 5. amber 6. malon 7. apple 8. formic* 9. benzoic* 10. lemon 9. Specify the 4 the causes of the greater activity tioefireов compared with esters in the reactions of SN : 1. interaction between carbon atom of carbon and sulphur, and more efficiently than with oxygen 2. mezomer positive effect of the R-S a group of less than the RO group* 3. alcoxid ion more easy going group than alkilsulfate ion 4. interaction between карбонильным atom of carbon and sulfur less efficiently than with oxygen* 5. mezomer positive effect of the R-About a group of less than RS group 6. and the positive charge on the карбонильном carbon in tioefireах higher than in the esters* 7. thiols sour alcohols, i.e. RS - groups are more easily leaving groups* 8. the positive charge on the carbonile carbon in tioesters less, than in the esters 10. Specify the 4 the causes of the greater activity chlorangadrate acetic acid compared with the acetic acid in the esterification reaction 1. positive charge on carbonil carbon chlorangadrate higher than that of the acid* 2. positive charge on carbonil carbon acids higher than that of the chlorangadrate 3. Cl - ion more poor basis, than OH - ion* 4. mezomer positive effect Cl - ion less than the OH-* 5. OH ion is a weak base, than Cl - ion 6. OH - ion well the outgoing group 7. Cl-ion well the outgoing group* 8. mezomer positive effect of OH - higher than the Cl - ion 11. Bring 4 example of condensation reaction in the body: 1. synthesis of α-amino acids 2. hydrolysis of carbohydrates 3. aldol splitting of α - amino acids* 4. aldol splitting carbohydrates* 5. the reaction between alcohols 6. the reaction tioesters with the formation of a new " links* 7. formation of β- hydroxi acids of α-oxo acids* 8. interaction of carboxylic acids from alcohols 12. Select 3 peculiarities of the structure of ureid of acids : 1. consist of 2 molecules of carbamide 2. contain the balance of urea ( carbamide )* 3. contain the balance of carboxylic acid* 4. are esters of carboxylic acids 21 5. are carbamid derivatives of carboxylic acids 6. are amides of carboxylic acids* 13. Select 3 features structure of ureidacides : 1. contain the balance of urea ( carbamide )* 2. are carboxylic acid amides 3. contain the balance of carboxylic acid* 4. consist of 2 molecules of carbamide 5. are carbamid derivatives of carboxylic acids* 6. are esters of carboxylic acids 3. Multi-level tests 1. Acid interact with alcohols. A. What is the name of the process of the interaction? a) condensation b) atsetilization c) eterification * d) hydrogenation e) dismutation B. What class of compounds when it is formed? a) carboxylic acids b) esters* c) nucleic acids d) amides e) cianhydrates C. What the mechanism of the reaction is typical for this process? a) nucleophilic substitution* b) electrophilic substitution c) electrophilic accession d) nucleophilic accession e) the radical replacement 2. In the exchange of substances in the body are involved representatives of the esters, amides, complex tioefireов, anhydrides and all of them during the hydrolysis to form one and the same class of organic compounds. A. The name of this class of compounds: a) saturated hydrocarbons b) ethylenic hydrocarbons c) heterofunctional connection d) acetylene hydrocarbons e) carboxylic acids* B. What is the mechanism of reactions is more characteristic for this group of compounds? a) nucleophilic substitution* b) electrophilic substitution c) electrophilic accession d) nucleophilic accession e) the radical replacement 22 C. Specify a factor contributes to the acceleration of the reactions coming to this mechanism? a) the presence of donor substituent in a molecule b) an acidic catalyst* c) cooling the reaction mixture d) the dilution of the solution e) reduction of the pressure in the system 3. In the body of coenzyme A activates carboxylic acids, turning them into reactionary capable esters of tiols. A. Why thioethers, and not the usual esters of selected nature as vectors of the acil groups? a) tioesters have more than the basic properties than conventional esters b) tioesters more active in the reactions of electrophilic substitution c) tioesters more active in the reactions of nucleophilic substitution* d) alkil sulfide-ions are bad leaving groups than alcoxid-ions e) alcoxid-ions esters are more easy to go groups than alkil sulfide-ions B. What is associated with their effectiveness in the reactions of metabolism? a) and the partial positive charge on the carbon atom in the esters higher than in tioesters b) and the partial positive charge on the carbon atom in tioesters above, than in the esters* c) and a partial negative charge on the carbon atom in tioesters higher than in the esters d) alcoxid ions easily replaced than alkil sulfide -ions e) esters easily hydrolysable by, than complex tioesters C. What caused this factor? a) RS-group gives more +M-effect than RO-group b) +M-effect RS-group and RO-groups are equal to each other c) RS-group gives no +M-effect d) RS-group provides a lower +M-effect than RO-group* e) RO-group gives no +M-effect 4. While studying the structure and properties of nucleic acids, fats and other biologically active compounds often found сложноэфирная connection. A. When interacting any connections it is formed? a) acids and amines b) acids and alcohols* c) aldehydes and alcohols d) ketones and amines e) ketones and alcohols B. Among the functional groups it is formed? a) aldehyde and hydroxyl b) ketone and hydroxyl c) carboxyl and amino group d) nitro group and carboxyl e) carboxyl and hydroxyl* 23 Estimation № Achievement C. The environment in which it hydrolyses? a) neutral b) in the environment of alkali metals c) acid and alkaline* d) in the environment of polar solvent e) in the environment of a non-polar solvent 5. The connection is the most important nitrogen the end product of metabolism and is a derivative of carbonic acid. A. What is the connection? a) ureid acides b) ureid of acid c) biuret d) guanidine e) urea* B. How derivative of carbonic acid it is? a) chlorangadrate b) diamide* c) amide d) ester e) anhydride In. What products it gives the hydrolysis? a) acid and ureid b) acid and alcohol c) carbon dioxide and ammonia* d) two acid e) acid and amin 9. Criteria for the evaluation of the current control The level of student knowledge Theoretical part: the Student gives the full, complete with from different literature sources reply on the topic of classes, the volume of knowledge goes beyond the limits of the program. Learned all the literary data, proposed in the programme and familiar with the additional literature. The job carries out in time and qualitatively. Actively participates in the discussion of themes and different views on the vision of the subject, confidently asserting its views on one or another debated issue. Do not make mistakes in writing formulas, equations and mechanisms of reactions, independently makes conclusions and make decisions. Actively and creatively participate in interactive teaching methods, the questions gives a comprehensive and well-grounded responses. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. Self-absorption of the topic and writing a synopsis. Summary compiled logically correct and includes a fully open questions text is presented systematically identifies the main literature and additional sources of information. Preparation of the abstract. In the collection of the material the student used, in addition to 24 Excellent «5» 96-100 1. Excellent «5» 91-95 2. the primary literature, additional sources of information (монографири, scientific and methodological articles and reports, information from Internet sources, materials of electronic бибилиотек, etc.), deeply analyzed and systematized. The theme of the essay is disclosed fully and completely, the text is presented in a logical sequence, conclusions are made correctly and feel the creative approach. Creative work. The student correctly filled with graphic organizers, tables, to their compilation approached creatively and reasonably. Training of scientific articles, theses and reports at: Students spend an independent experimental work in SSA, designed in work-book, independently spent their discussion and processed statistical data, made a reasonable, logical faithful, educated conclusions. Of educational and scientific literature, the results of scientific-research works, articles, theses and monographs, as well as other sources of information a student gathered theoretical material on a theme of work, analyzed and systematized all the literary data. Prepared by the article, thesis or report are based on the scientific results of the self-experimental work and the analysis of literary data collected by the student. Practical part:Mastered all the practical skills, apply the received theoretical knowledge in carrying out of experimental works. Without errors carries out step-by-step implementation of reactions, correctly writes equations of the reactions, substantiate and make the right conclusions. The analytical part: Creativity and the right thinks when solving situational tasks and tests give accurate and logical answers. Theoretical part: a Student gives a complete answer on the topic of classes, the volume of knowledge of the limits of the program. Learned all the literary data, proposed in the programme and familiar with the additional literature. The job carries out in time and qualitatively. Actively participates in the discussion of themes and different views on the vision of the subject, confidently asserting its views on one or another debated issue. Do not make mistakes in writing formulas, equations and mechanisms of reactions, independently makes conclusions and make decisions. Actively and creatively participate in interactive teaching methods, the questions gives a comprehensive and well-grounded responses. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. Self-absorption of the topic and writing a synopsis. Summary compiled logically correct and includes a fully open questions text is presented systematically identifies the main literature and additional sources of information. Preparation of the abstract. In the collection of the material the student used, in addition to the primary literature, additional sources of information (монографири, scientific and methodological articles and reports, information from Internet sources, materials of electronic бибилиотек, etc.), deeply analyzed and systematized. The theme of the essay is disclosed fully and completely, the text is presented in a logical sequence, conclusions are made correctly and feel the creative approach. Creative work. The student correctly filled with graphic organizers, tables, to their compilation approached creatively and reasonably. Training of scientific articles, theses and reports at: Students spend an independent experimental work in SSA, designed in work-book, independently spent their discussion and processed statistical data, made a reasonable, logical faithful, educated conclusions. Of educational and scientific literature, the results of scientific-research works, articles, theses and monographs, as well as other sources of information a student gathered theoretical material on a theme of work, analyzed and systematized all the literary data. Prepared by the article, thesis or report are based on the scientific results of the self-experimental work and the analysis of literary data collected by the student. Practical part:Mastered all the practical skills, apply the received theoretical knowledge in 25 Excellent «5» Theoretical part: a Student gives a complete answer on the topic of classes, the volume of knowledge in the programme. Learned all the literary data, proposed in the programme and familiar with the additional literature. The job carries out in time and qualitatively. Actively participates in the discussion of themes and different views on the vision of the subject, confidently asserting its views on one or another debated issue. Do not make mistakes in writing formulas, equations and mechanisms of reactions, independently makes conclusions and make decisions. Actively and creatively participate in interactive teaching methods, the questions gives a comprehensive and well-grounded responses. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. Self-absorption of the topic and writing a synopsis. Summary compiled logically correct and includes a fully open questions text is presented systematically identifies the main literature and additional sources of information. Preparation of the abstract. In the collection of the material the student used, in addition to the primary literature, additional sources of information (монографири, scientific and methodological articles and reports, information from Internet sources, materials of electronic бибилиотек, etc.), properly analyzed and systematized. The theme of the essay is disclosed fully and completely, the text is presented in a logical sequence, conclusions are made correctly and feel the creative approach. Creative work. The student correctly filled with graphic organizers, tables, to their compilation approached creatively and reasonably. Practical part:Mastered all the practical skills, apply the received theoretical knowledge in carrying out of experimental works. Without errors carries out step-by-step implementation of reactions, correctly writes equations of the reactions, substantiate and make the right conclusions. The analytical part: Correctly solves situational tasks and tests give accurate and logical answers. Good «4» carrying out of experimental works. Without errors carries out step-by-step implementation of reactions, correctly writes equations of the reactions, substantiate and make the right conclusions. The analytical part: Creativity and the right thinks when solving situational tasks and tests give accurate and logical answers. Theoretical part: a Student gives a complete answer on the topic of classes, the volume of knowledge in the programme, is responsible confidently. Learned all the literary data, proposed in the programme and familiar with the additional literature. Job performs on time and correctly. Actively participates in the discussion of themes and different views on the vision of the subject, confidently asserting its views on one or another debated issue. In writing formulas, equations and reaction mechanisms, allows for minor errors. Actively participating in the interactive methods of training, to questions gives the most part correct answers. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. Self-absorption of the topic and writing a synopsis. The summary includes all open questions, the text is presented systematically identifies the main literature and additional sources of information. Preparation of the abstract. In the collection of the material the student used, in addition to the primary literature, additional sources of information (монографири, scientific and methodological articles and reports, information from Internet sources, materials of electronic бибилиотек, etc.), analyzed and systematized. The theme of the essay is opened, the conclusions are made correctly. Creative work. The student correctly filled with graphic organizers, the tables are filled in correctly and completely. Practical part:Mastered all the practical skills, apply the received theoretical knowledge in carrying out of experimental works. Without errors carries out step-by-step implementation of reactions by writing the equations allows 1-2 small mistakes, substantiate and make the right conclusions. 86-90 3. 81-85 4. 26 The analytical part: Correctly solves situational tasks and tests give accurate and logical answers. Good 4» 76-80 5. The analytical part: Correctly solves situational tasks and tests give accurate and logical answers. Theoretical part: the Amount of the student's knowledge in the programme, is responsible confidently. Learned all of the main literary sources. Job performs on time. Actively participates in the discussion of themes and different views on the vision of the subject. In writing formulas, equations and reaction mechanisms, allows for 2-3 errors. Participating in the interactive methods of training, to questions gives greater part of the correct answers, but some of the answers may not justify. Good «4» The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. Self-absorption of the topic and writing a synopsis. The summary includes all open questions, the text is presented systematically identifies the main literature and sources of information. Preparation of the abstract. In the collection of the material the student used, in addition to the primary literature, additional sources of information (монографири, scientific and methodological articles and reports, information from Internet sources, materials of electronic бибилиотек, etc.), analyzed and systematized. The theme of the essay is opened, the conclusions are made correctly. Creative work. The student correctly filled with graphic organizers, the tables are filled in correctly and completely. Practical part: Mastered all the practical skills, apply the received theoretical knowledge in carrying out of experimental works. Without errors carries out stepby-step implementation of reactions by writing the equations allows for 2-3 small mistakes, substantiate and make the right conclusions. The analytical part: Correctly solves situational tasks and tests, but is difficult to substantiate the answer. Theoretical part: the Amount of the student's knowledge is 60-70% of the programme. Learned the main literary sources. Has an idea about the structure and properties of the considered compounds, further questions the answers are incomplete. Participating in the interactive methods of training, to questions gives 66-70 Satisfact orily «3» 71-75 6. 7. Theoretical part: the Amount of the student's knowledge in the programme, is responsible confidently. Learned all of the main literary sources. Job performs on time. Actively participates in the discussion of themes and different views on the vision of the subject. In writing formulas, equations and reaction mechanisms, allows 1-2 errors. Actively participating in the interactive methods of training, to questions gives greater part of the correct answers, but also allows for minor errors. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. Self-absorption of the topic and writing a synopsis. The summary includes all open questions, the text is presented systematically identifies the main literature and sources of information. Preparation of the abstract. In the collection of the material the student used, in addition to the primary literature, additional sources of information (монографири, scientific and methodological articles and reports, information from Internet sources, materials of electronic бибилиотек, etc.), analyzed and systematized. The theme of the essay is opened, the conclusions are made correctly. Creative work. The student correctly filled with graphic organizers, the tables are filled in correctly and completely. Practical part:Mastered all the practical skills, apply the received theoretical knowledge in carrying out of experimental works. Without errors carries out step-by-step implementation of reactions by writing the equations allows 1-2 small mistakes, substantiate and make the right conclusions. 27 greater part of the right answers, but the answers allows a number of errors The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. Self-absorption of the topic and writing a synopsis. The summary includes all open questions, but there is no systematic in the presentation of text, specified the main literature and sources of information. Preparation of the abstract. The student uses only the main sources of literature, analyzed and made some conclusions. The topic of the abstract disclosed, but there are some drawbacks. Creative work. Student filled with graphic organizers, but difficult in the justification of the responses, when filling in tables admitted some disadvantages. Practical part: Mastered practical skills, but allows some errors. Carries out step-by-step implementation of reactions by writing the equations allows 3-4 mistakes, but difficult to substantiate and conclusions. The analytical part: When solving situational tasks and tests allow some errors. Theoretical part: the Amount of the student's knowledge is 55-60% of the programme. Learned the main literary sources. Has an idea about the structure and properties of the considered compounds, further questions the answers are incomplete. Inactive in interactive methods of training, to some of the questions the answers, but the answers makes mistakes. Satisfactorily «3» 61-65 8. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. Self-absorption of the topic and writing a synopsis. The summary includes all open questions, but there is no systematic in the presentation of text, specified the main literature and sources of information. Preparation of the abstract. The student uses only the main sources of literature, analyzed and made some conclusions. The topic of the abstract disclosed, but there are a number of shortcomings. Creative work. Student filled with graphic organizers, but mistakes have been made, does not justify the answers, when filling in tables admitted shortcomings. Practical part: Mastered practical skills, but allows some errors. Is wrong with the carrying out step-by-step implementation of reactions by writing the equations allows 3-4 gross errors, but difficult to substantiate and conclusions. The analytical part: When solving situational tasks and tests allow some errors. Theoretical part: the Volume of knowledge of a student is 50-55 per cent of the programme. For additional questions, not answers. Partially used literature. Has an idea about the structure and properties of the considered compounds, in response to the questions allows a lot of mistakes. Inactive in interactive teaching methods, the questions gives a superficial answers. Satisfactorily «3» 55-60 9. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. Self-absorption of the topic and writing a synopsis. In summary all the questions do not disclosed, there is no systematic in the presentation of the text, not specified the main literature and sources of information. Preparation of the abstract. The student uses only the main sources of literature, but there is no systematic and analysis. The theme of the essay is not disclosed. Creative work. Student filled with graphic organizers, but may not work alone, can not justify the answers, when filling in tables admitted shortcomings. Practical part: Mastered practical skills in part, cannot independently carry out laboratory work. Is wrong with the carrying out step-by-step implementation of reactions by writing the equations admits many gross errors, does Not make independent conclusions. The analytical part: When solving situational tasks and tests, there is no independence. 28 Неудовлет unsatisfactory ьно «2» unsatisfactory «2» 50-54 10. 36-40 13 unsatisfactory unsatisfactory «2» 41-45 12 Theoretical part: the Volume of knowledge of a student is 30-40% of the programme. Is not clear on the theoretical aspects of the topic. For additional questions, not answers. Not mastered the basic literature. Has a slight idea about the structure and properties of the considered compounds, but practically does not respond to questions. Does not perform in time job data in the lesson. Does not participate in interactive teaching methods. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. For the abstract and the abstract assembled a small stuff, but a lot of mistakes when writing. Graphic organizers are not compiled. Practical part: Mastered practical skills in part, cannot independently carry out laboratory work. Cannot independently carry out step-by-step implementation of reactions by writing the equations admits many gross errors, does Not make independent conclusions. The analytical part: can't decide situational tasks and tests. Theoretical part: the Volume of knowledge of a student is 20-30% of the programme. Is not clear on the theoretical aspects of the topic. For additional questions, not answers. Not mastered the basic literature. Has a slight idea about the structure and properties of the considered compounds, but practically does not respond to questions. Does not perform in time jobs, data on the lesson. Does not participate in interactive teaching methods, the questions can't answer. By the end of session acquires some ideas for names, formulas and conclusions of the studies. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. For the abstract and the abstract assembled a small stuff, but a lot of mistakes when writing. Graphic organizers are not compiled. Practical part: Mastered practical skills in part, cannot independently carry out laboratory work. Cannot independently carry out step-by-step implementation of reactions by writing the equations admits many gross errors, does Not make independent conclusions. The analytical part: can't decide situational tasks and tests. Theoretical part: the Volume of knowledge of a student is 20% of the programme. Is not clear on the theoretical aspects of the topic. For additional questions, not answers. Not mastered the basic literature. Has no idea about the structure and properties of the considered compounds, does not answer the questions. Does not perform in time jobs, data on the lesson. Does not participate in interactive teaching methods, the questions can't answer. By the end of session acquires some ideas for names, formulas and conclusions of the studies. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. For the abstract and the abstract assembled a little material, but not written. Graphic organizers are not compiled. Practical part: Mastered practical skills in part, cannot independently carry out laboratory work. Cannot independently carry out step-by-step implementation of reactions do not know how to write equations,does not make independent conclusions. The analytical part: can't decide situational tasks and tests. Theoretical part: the Volume of knowledge of a student is 10% of the programme. Is not clear on the theoretical aspects of the topic. For additional questions, not answers. Not mastered the basic literature. Has no idea about the structure and properties of the considered compounds, does not answer the questions. Does not perform in time jobs, data on the lesson. Does not participate in interactive teaching methods, the questions can't answer. By the end of session acquires some ideas for names, formulas and conclusions of the studies. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. For the abstract and the abstract assembled a little material, but not written. Graphic organizers are not compiled. 29 Practical part: Mastered practical skills in part, cannot independently carry out laboratory work. Cannot independently carry out step-by-step implementation of reactions do not know how to write equations,does not make independent conclusions. The analytical part: can't decide situational tasks and tests. unsatisfactory «2» 31-35 14. The theoretical part of the The SIW The practical part of the The analytical part of Theoretical part: could not answer definitely. Has no idea on the theoretical aspects of the topic. Not mastered the basic literature. Has no idea about the structure and properties of the considered compounds, does not answer the questions. Does not perform in time jobs, data on the lesson. Does not participate in interactive teaching methods, the questions can't answer. By the end of session acquires some ideas for names, formulas and conclusions of the studies. The form of independent work: self-learning topics, preparation of the abstract, scientific articles, theses and reports. For the abstract and the abstract assembled a little material, but not written. Graphic organizers are not compiled. Practical part: Mastered practical skills in part, cannot independently carry out laboratory work. Cannot independently carry out step-by-step implementation of reactions do not know how to write equations and not draw conclusions. The analytical part: can't decide situational tasks and tests. 96-100 44-45 91-95 42-43 86-90 38-39 81-85 36-37 76-80 33-34 71-75 31-32 66-70 29-30 61-65 27-28 55-60 26-27 31-54 12-25 0-30 0-11 9-10 19-20 8-9 18-19 8-9 18-19 7-8 17-18 7-8 16-17 6-7 15-16 6-7 14-15 6-7 13-14 6-7 10-11 4-5 6-9 0-4 0-6 24-25 23-24 22-23 21-22 20-21 19-20 17-18 15-16 13-15 9-15 9 10. Literature Summary: 1. N.A.Tyukavkina. Bioorganic Chemistry, Moscow, 2004 2. N.A.Tyukavkina. A guide to laboratory work on Bioorganic Chemistry, Moscow, 1999 General: 1. Sh.U.Abdullaev, A.D.Dzhuraev, U.A.Boltabaev and other management to practical training in Bioorganic Chemistry, Tashkent, 1982 1. Shris Conoley, Phil Hills. Chemistry. London, 1998. 2. B.N.Stepanenko. Organic Chemistry. 1980 3. Z.Gauptman. Organic chemistry, M. 1983 5. P. Sykes - Mechanisms of Reactions in Organic Chemistry, 1977 6. Yu.A.Rayls. Fundamentals of Organic Chemistry, M.1993. 7. Sources of information from the Internet: 30 www.tma.uzsi.net.; www mail.ru; http:// www. doctor. Ru / medinfo; http://medinfo. home. ml. org. ; Http://www.bankreferatov.ru; http://www. 5 ballov.ru; http://www. referat. Ru C: \ Documents and Settings \ Administrator \ My Documents \ Organik_KIMYO C: \ Documents and Settings \ Administrator \ My Documents \ Tutorial organic himii_files www.tma.uzsi.net. Application to the method of «Brainstorming»: Appendix №1 Questions blitz-poll: Theacher 1. Classification and nomenclature of carboxylic acids, carboxylic acids of an organism 2. Types of reactions of carboxylic acids 3. How to increase the reactivity of carboxylic acids? 4. What substaces come into reaction of carboxylic acids? 5. The product of the reaction with alcohols 6. The product of the reaction with tiols 7. The product of the reaction with ammonia 8. The product of the reaction with phosphorus anhydride 9. The product of the reaction with sodium hydroxide 10. The product reacts with phosphoric acid 11. The product of the reaction with phosphorus chloride 12. The product of the reaction with coenzyme A 1З. The environment in which hydrolysable by esters? 14. The environment in which hydrolysable by anhydrides? 15. The environment in which hydrolysable by amide connection? 16. The environment in which hydrolysable by halogenanhydrates connection? 17. As a proven mechanism of the reaction of the esterification? 18. The product of hydrolysis of fats 19. What are the consequences of electron donor deputies on the reactivity of carbon acids? 20. What are the consequences of электроноакцепторные deputies on the reactivity of carbon acids? Student Answer questions quickly and briefly Appendix № 2 Small group The number of group members and experts Problems to be solved in small groups 31 1-group 4 (1 the expert) 2-group 4 (1 the expert) 3-group 4 (1 the expert) 4- group 4 (1 the expert) 1. The reactionary centers in molecules carboxil containing connections. The reactivity and the factors influencing it. The General scheme of the mechanism of nucleophilic substitution 1. The reaction of formation of esters and tioefireов carboxylic acids and their mechanism 1. Reactions of education and halogen anhydrides of carboxylic acids and their mechanism 1. Reactions of education amides, hydrazides of carboxylic acids and it’s mecchanisim Appendix № 3 Independent work of the student, designed as an abstract and (or) the presentation. 32